1000/1000
Hot
Most Recent

Arsenic (As) removal from drinking water is of critical importance because in inorganic form As is highly toxic to all life forms, is a confirmed carcinogen and is of significant environmental concern. As contamination in drinking water alone threatens >150 million people all over the world. In order to address the increasing demand of As-free water; innovative nanofiltration (NF) strategies for As-removal have been advanced. This article presents a critical overview of the current status of nanomaterial-facilitated NF membranes identifying key deficiencies needs and challenges, to stimulate future research and progress. Finally, the future prospects and trends are also highlighted.
Arsenic (As) removal is of major significance because inorganic arsenic is highly toxic to all life forms, is a confirmed carcinogen, and is of significant environmental concern.
In recent years, the source of pure water is continuously diminishing due to groundwater decline and depletion, climate change, poor resource management, and environmental pollution. In addition to this, the increasing rate of the world population, ~80 million per year, results in a growing demand for water by 64 billion cubic meters per annum [1][2]. The major pollutants of water are organic dyes, radioactive metals, heavy metals, and oxyanions of metals (CrO42−, AsO43−, SeO32−, etc.). Among the pollutants, the toxicity of arsenic oxyanions is very serious and unique as they originate into the water body due to natural phenomena—either from natural soil sources or from anthropogenic sources. Moreover, they are carcinogenic. The consequence of consumption of arsenic-contaminated drinking water has evolved as one of the major health hazards in recent times. High concentration of arsenic (As) in drinking water may initially cause skin disease and eventually can turn to cancer and is of the utmost concern to public health [3]. The regulatory agencies around the globe such as World Health Organization (WHO) [4], US Environmental Protection Agency (USEPA) [5], Health Canada [6], and European Union (EU) [7] have set maximum limits on arsenic in drinking water to 0.01 mg/L to ensure the safe consumption of arsenic-contaminated drinking water to protect people from diseases. People of different parts of Bangladesh, West Bengal [8] and some other parts of India [9], Cambodia [10], Inner Mongolia of China [11][12], Iranian Kurdistan [13], Thailand [14], Eastern Croatia [15], Mexico [16], El Salvador, Peru, Nicaragua [17], northern Afghanistan, northern Mali and Zambia in Africa [18], and Vietnam [10] are living under the dire threat of arsenic toxicity [19][20]. At present, there are 628.5, 2281.2, and 2071.8 million people living in 21, 29, and 26 countries that are in the categories of the low, lower medium, and upper-medium groups respectively [21]. Among them, the poisoning effect of arsenic is most severe in the areas of Bengal Delta (Bangladesh, Nepal, and West Bengal), where concentrations of dissolved arsenic exceed over 200 μg/L. More than 3.57 billion people living in Bangladesh, Vietnam, Pakistan, India, Afghanistan, Nepal, Mali, Nigeria, etc. are at risk, and due to the arsenic-contaminated drinking water, at least 100 million people are already affected [20]. Consequently, effective purification methods for arsenic removal from water is of critical importance and of profound societal value. However, due to the low socio-economic status of the major affected countries, they cannot afford expensive and large-scale treatments to remove arsenic from drinking water to the acceptable levels (10 ppb, as recommended by WHO and US EPA). Therefore, development of facile and cost-effective methods, which are easy to handle and conveniently applied in large scale at household and community levels for the remediation of arsenic from contaminated groundwater is of the highest priority.
Considering the societal value and critical importance, both traditional and different emerging technologies based on chemical, biochemical/biological/biosorption, and physico-chemical treatment processes for arsenic removal from contaminated drinking water have been developed and applied [22]. Many sustainable and naturally abundant materials including waste rice husk [23]; iron-based adsorbents such as iron oxy-hydroxides including akaganeite (β-FeOOH), goethite (α-FeOOH), lepidocrocite (γ-FeOOH), ferrihydrites (Fe10O14(OH)2), iron-based layered double hydroxides (LDHs), iron oxy-hydroxides doped activated carbon, and iron oxy-hydroxides doped graphene oxide [24][25]; and activated carbon [26] have been examined as efficient adsorbents for As removal. Recently, some novel materials including cellulose-based fibers [27][28], metal organic framework (MOF) [29], and hydrogel [30] have also been explored. Several of these As removal materials and techniques have also been implemented practically in the affected rural areas. The main concern of all the effective processes is mainly related to cost, both initial and operational, as the arsenic problem is mostly in developing or underdeveloped countries and areas. In a report by the World Bank, it has been stated that especially low (US$ < 1025), lower medium (US$ 1026–4035), and upper-medium (US$ 4036–12,475) income countries are facing challenges to reduce arsenic below the guideline values due to their limited economic capacities [31]. Therefore, there is a drive for continuous improvement in the existing removal method as well as introducing new technologies.
The developed methods for ‘As’ removal include chemical precipitation, adsorption, coagulation, electrocoagulation, ion exchange, oxidation, and membrane filtration. The membrane filtration is a boosting technology in the field of separation technology. In recent years, potable water purification using membrane technology has become attractive worldwide due to its simplicity and versatility, increased stringency of regulations, decreasing costs, and increasing commercial availability of a variety of membranes. It does not require any additives or chemicals, and only minimal amounts of energy. Membrane technology is a clean technology, where the separation process is carried out solely on the basis of molecular size and use of additives is not necessary and has the potential to boost the product quality and bring down the overall production costs. Currently, it is considered superior to other separation methods because of its easy operation and no sludge formation. However, there are liquid wastes containing dissolved solids (concentrates) in the water rejected by membrane systems. The disposal of waste streams generated by removing arsenic from drinking water is of concern, because As can be highly mobile and has the potential to leach back into the ground and surface waters. The residual disposal options are highly site-specific. These wastes can be discharged to a receptor body if they comply with the regulations; on the contrary, they must be submitted to treatments such as chemical precipitation and coagulation-sedimentation-filtration with the generation of solid wastes (muds or sludges). In the case of semiliquid wastes, they must be submitted to thickening and dewatering processes [32]. Though, different effective, reliable, and sustainable methods of As waste disposal have been developed and proposed, recent emphasis has been given on stabilization/solidification (S/S) technologies, which are currently used to treat industrial wastes containing As [33].
The four most popular pressure driven membrane filtration processes for liquid separation are respectively: (i) reverse osmosis, (ii) nanofiltration, (iii) ultrafiltration, and (iv) microfiltration—in ascending order of size of the particle that can be separated. The microfiltration is a low pressure-driven filtration process for arsenic removal; however, these membranes are not highly efficient for arsenic removal as they are based on the pore flow model and allow multivalent ion to pass through the membrane pores. On the other hand, reverse osmosis membrane can efficiently remove arsenic; however, it is energy intensive and its operational cost is higher. Nanofiltration has attracted considerable attention from researchers due to its high efficiency with lower operational cost [34]. The separation process in nanofiltration (NF) membrane depends both on size sieving and Donnan exclusion, and efficiently rejects multivalent dissolved ions of water. These properties make it a highly suitable separation technology for arsenic removal [35].
Different nanofiltration membrane fabrication method was developed focusing on the resulting pore size (1–10 micron) of the membrane. The most common fabrication method for making polymeric nanofiltration membranes is interfacial polymerization. It also discussed in the previous section that the interfacial polymerization method has been used to make different types of NF membrane for arsenic removal. In this process, an ultrafiltration membrane substrate is used to support the selective layer of the membrane. It is formed in a TFC structure. The thin layer is formed by a reaction between two reactive monomers. Several different types of reactive monomers/prepolymers including bisphenol-A (BPA), tannic acid, m-phenylenediamine (MPD), diethylenetriamine (DETA), triethylenetetramine (TETA), tetraethylenepentamine (TEPA), piperazidine (PIP), polyvinylamine reacting with trimesoyl chloride (TMC), or isophthaloyl chloride have been successfully employed using IP process to form the thin active film layer in TFC-NF [36][37][38][39]. Polyhexamethylene guanidine hydrochloride (PHGH) has been successfully used as a monomer to form the active layer using the IP method to prepare NF membrane with excellent bacteria inhibition characteristics [40]. Wang et al., reported a simple elegant method for the fabrication of an efficient TFC-NF membrane with a crumpled polyamide (PA) layer via IF on a single-walled carbon nanotubes/polyether sulfone composite support membrane loaded with sacrificial nanoparticles [41]. Recently, a novel approach to design TFC membranes with ultrahigh permeance using hydrogel assisted IF has been advanced, which is facile, and enables cost-efficient and scalable manufacturing [42].
Regardless of their advantages, only a limited number of polymeric NF membranes can be prepared using interfacial polymerization, which limits several key improvements of the membrane such as hydrophilicity, antifouling property, chemical resistance, longer lifespan, and rejection efficiency. Though, incorporation of polymer in the membrane/polymer solution prior to the interfacial reaction often helps to improve the property of the membrane. The recent progress in the NF membrane largely focuses on fabricating the thin-film nanocomposite (TFN) membrane by adding nanoparticles [43]. TFN-NF incorporating additives such as nanoparticles in the active layer has also been promoted. TFC-NF membranes using IP technique and based on polyetherimide (PEI) modified with amine-functionalized silica NPs has been employed to improve the mechanical and thermal stability of the membranes [44]. The introduction of inorganic salt (e.g., calcium chloride (CaCl2) dissolved in aqueous phase and organic acids with different structures such as ascorbic acid, citric acid, malic acid, and acidic strengths were studied during the fabrication and used as an additive during the IP process [45][46]. Metal-alkoxide (e.g., titanium tetraisopropoxide, bis-(triethoxysilyl)-ethane phenyltriethoxysilane) assisted IP has also been reported for the synthesis of inorganic-polyamide nanocomposite membranes to improve the permeability performance [47]. NF membranes, consisting of a composite barrier layer prepared by interfacial polymerization of polyamide around the ultra-fine cellulose nanofibers (CN) layer in a thin-film nanofibrous composite (TFNC) scaffold were demonstrated [48]. However, there are some problems associated with the incorporation of nanoparticle in the IP membrane such as poor dispersion and agglomeration of particles. A variety of surface modifications [49], zwitterionization [50][51], and hybridization [52] during IF process have been employed to enhance the performance of NFs.
The NF membrane is preferred to be very thin for getting high water flux. The porous support layer is the unavoidable part of the TFC NF membrane to support the thin selective upper layer. The support layer is prepared using the phase inversion process. Therefore, it increases the steps in the membrane fabrication process. Besides, usually a very toxic solvent is used in the phase inversion process to make the ultrafiltration membrane support. To avoid some of the critical disadvantages, electrospun membrane has been developed to replace the conventional membrane support system; as the transport properties of the membrane is enhanced by interconnected pore structure, which permits a shorter path for water transport [53][54]. In addition, the electrospun nanofiber membrane can be thin with good mechanical properties [55] as discussed below.
Electrospinning is a flexible technique for making nanofibrous porous membranes for different applications such as wastewater treatment, desalination, and filtration [56][57]. Several advantages like low start-up cost, high surface area to volume ratio, 3D interconnected pore structure, more contacting surface (the fiber surface), high strength, and feasibility in making well dispersed mixed matrix membrane make it a viable fabrication technique for membrane preparation. In addition, the electrospun membrane can be prepared from most of the major classes of the polymer [58]. The polymer is taken in solution and the filler materials like nanoparticle are dispersed to the polymer solution prior to the electrospinning and then the polymer solution is loaded in the syringe and placed in the syringe pump as shown in Figure 1. A high voltage is applied between the stationary or rotating collector and the spinneret to make the fiber from the jet solution. The nanofiber is accumulated in the collector to make the nanofibrous membrane [59]. The porosity of the membrane depends on the operational parameters such as voltage, feed rate, the distance between collector and spinneret, operation time, and solution property. By tailoring the solution, property and operational parameters desired membrane morphology can be obtained. There is room for more research to make a versatile NF membrane using electrospinning technology.
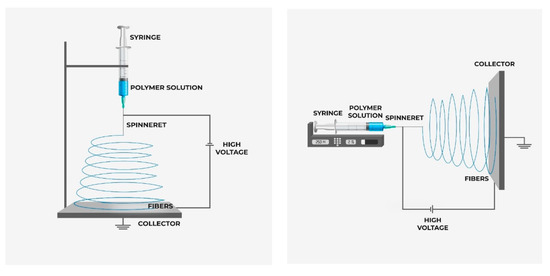
Figure 1. Schematic diagram of electrospinning process (vertical and horizontal).
Graphene oxide (GO) has different functional groups (hydroxyl, carboxyl, epoxide, and C=C) on the surface, which makes it a preferred and desired nanomaterial to use solely and as a filler material in different types of membranes for the modification and enhancing the properties of the membrane. The addition of GO in the membrane enhances the water molecules transportation through the membrane by forming the interconnected nanochannels [60]. GO is also hydrophilic in nature due to the presence of the hydrophilic group as well as has great antifouling, antibiofouling, and antibacterial properties [61][62][63]. The wide attention paid to GO is shown in Figure 2 as well as the application in various fields. GO has been used solely as the adsorbents for the removal of arsenic, where the efficiency has been found 100% for arsenate of a 20-mg/L concentration [64]. In the same research, 100% arsenate removal was also achieved by a nanocomposite of GO and 3-aminopyrazole. Mostly, the graphene-based materials used as nanocomposites are consist of metal oxides or organic/inorganic compounds with GO or reduced graphene oxide (rGO). Iron oxides (mostly Fe3O4), copper oxides, etc. were used with GO or rGO in the nanocomposites [65][66][67][68][69], some organic compounds such as epoxy, aminopyrazole, EDTA-Chitosan, Imino-Thiobiuret, etc. [64][70][71][72] and inorganic compounds like LD-Hydroxide, silica, etc. [73][74] have been used with GO or rGO to prepare the nanocomposites as well. The arsenate removal efficiency of these nanocomposites is in the range of 80–100%. The hydrothermal treatment, solid state dispersion, and aqueous thermal treatment have been used for the synthesis of these nanocomposites.
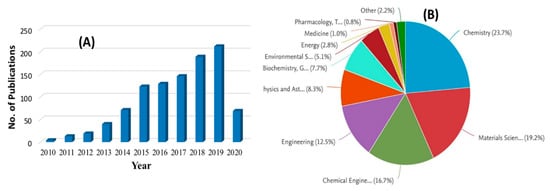
Figure 2. (A) The number of publications and (B) application of graphene oxide (GO) in various fields based on the keyword “graphene oxide nanocomposite membranes” in Scopus database (data collected on 14.04.2020).
Although NF membranes offer several unique properties for application in the arsenic removal technology, large scale application is still in the premature stage because of several obstacles such as lack of research on membrane materials, low durability of membranes, and water flux declination with time. Most of the research groups focused on the application of commercially available NF membranes rather than synthesizing new membrane specialized on arsenic removal. Though arsenite is more toxic than arsenate, most of the research reports were focused on the arsenate removal as the removal process is easier due to the Donnan exclusion effect of membranes. Most of the current state-of-the-art commercial membrane technology for NF is based on a cross-linked polyamide membrane chemistry. The most commonly employed amines are piperazine, while trimesoyl chloride (TMC) is the most common acid chloride, leading to a cross-linked three-dimensional structure with residual end-groups as amine and COOH. In TFC, the barrier layer has a thickness of ca. 200 nm, and is generally an integral part of a three-layered composite membrane. In such a three-layered composite membrane, typically the bottom layer is a polyethylene terephthalate (PET) reinforcing web to provide mechanical support, followed by an asymmetric polysulfone (PS) layer with surface porosity in the tens of nanometers, and finally the polyamide barrier layer for AS removal. NF polyamides are negatively charged at practical operating conditions, a product of the balance between dissociated free acid and amine end groups. These negatively-charged membrane surfaces are more susceptible to membrane fouling activity. The swelling tendency and lower mechanical strength of the membranes also decrease the durability of NF membranes for As removal as well as the membrane fouling decreases the water flux with time. Although, the mixed matrix membrane and thin film nanocomposites could be the solution to the existing problems, extensive research is required to establish a mixed matrix membrane for efficient arsenic removal. In addition, a NF membrane synthesis process for As removal has not been explored yet, instead, mostly interfacial polymerization has been used to prepare the NF membranes for arsenic removal. Another hurdle is the reduced possibility of ensuring suitable pore size and distribution in large-scale production.
In recent years, approaches for the establishment of a membrane-based arsenic removal technique is a highly active research field. Among various membrane types, the NF membranes are leading because of their impressive arsenic rejection with high flux and low-pressure operation. In this review, recent developments of the NF membranes for arsenic removal have been discussed and our comparative analysis reveals several insights for their structure-property-performance relationship and their practical implementation. It appears that the current studies mostly focused on commercial TFC membranes. The commercial NF membranes, however, are not effective for the successful removal of both AsIII and AsV. Moreover, arsenic rejection and water flux have not been studied extensively. Although new advanced NF membranes including thin film nanocomposite (TFN)/mix-matrix NF membranes are emerging and being gradually introduced for arsenic removal; however, the research is still at a premature level. Future research should focus on introducing new membrane material, novel low cost, an environmentally benign membrane synthesis process to fabricate advanced TFC/TFN membranes in order to improve the water flux, rejection of both AsV and AsIII, and decrease the membrane fouling. By focusing on all the mentioned features, NF membranes have the potential to be the most efficient and economic arsenic removal method and it would be possible to adopt it for water treatment in the affected areas, both at a household and community level.
An exciting number of next-generation polymer materials are currently being investigated that have the potential to overcome the current limitations and supersede the energy and separation capabilities of industry standard polyamides NFs. Among them, block copolymers (BCP) and liquid crystals (LC) membranes have demonstrated a remarkable ability as NF membranes [75]. BCP is one of the most versatile and tailorable materials. Not only their chemical compositions can be tailored over a wide range of composition levels, but also varying processing criterion such as the solvent interactions can also be utilized as a tool to tailor NF membrane porosity and morphology. Moreover, in BCP-based NFs, additional functionalities such as antifouling properties [76], pH responsiveness [77], and thermal responsiveness [78] could be easily introduced using post-processing steps. In such stimuli responses of NFs membranes, the flux of the membranes could be altered by both pH and temperature. The flux in LC membranes are reported to be orders of magnitude below those of BCP and polyamide membranes [75]. In the LC space, the potential of using polymerizable lyotropic liquid crystal (LLC) assemblies as NF membranes has been demonstrated [79]. The biggest challenge facing BCP and LC self-assembled membranes is the cost of materials, compared to conventional materials used for NF applications. The processability of these systems, particularly BCPs are readily tailorable to large-scale manufacturing and with appropriate investment in scale-up technologies the gap could be overcome in the future for wider applications.
Recently, 2D materials, specifically graphene, have attracted significant attention as potential NF membrane material because they hold a promise of providing minimum resistance to water transport. Graphene, by definition is only a single atomic layer thick, and is a truly 2D material. Computational studies have demonstrated high predicted rates of water transport and salt rejection through nanoporous graphene membranes [80]. The aromatic rings of the honeycomb lattice of graphene are too small for the passage of molecules such as salts, water, and even gases. Thus, creation of intentional nanopores of requested size and uniform distribution for water transport has been developed as a potential approach to make an effective NF membrane [81]. Graphene sheets can be transformed into membranes in a variety of ways: (i) composite blends with polymers or other matrix structures, (ii) stacks of sheets, or (iii) truly 2D single layer or few layer continuous membranes [74]. Though considered ideal, graphene presents greater challenges in (i) large area scalability, (ii) generation of monodisperse distribution of optimally sized pores, (iii) the complexity of processing, and (iv) cost of production. Pore uniformity and defect management are the two major factors limiting the accelerated development of nanoporous graphene membranes, even for high-end applications. To mitigate such problems, graphene oxide (GO) membranes formed in layer-by-layer (LBL) GO structures that behaved quite similar to commercial NF, have been attempted successfully. GO flakes can be prepared by chemical oxidation of abundantly available and sustainable graphite followed by exfoliation to release individual or few layer nanosheets into aqueous suspension [82][83]. The sheets can range in size from a few hundred nanometers up to a few micrometers. Film formation from such dispersions becomes much more straightforward, and consequently, GO membranes used in water filtration are generally composed of layered GO flakes coated onto a microporous support surface. The flow profile in a GO laminate film is one of percolation, with lateral flow along hydrophobic channels combined with periodic perpendicular transport through spacing between adjacent nanosheets, as well as holes of imperfection within sheets. While many GO membranes have provided ultrahigh water permeability, their selectivity for ionic solutes, thus far, have not been competitive against current NF membranes, which may result from defects introduced during the casting of the thin films.
Biomimetic membrane technology-drawing inspiration from nature’s own ways of transporting and purifying water has recently been a motivation for development of more efficient separation membranes to purify water faster and more efficiently than ever before. Over millions of years, nature has evolved remarkable water channel proteins, e.g., aquaporins, which are crucial for life in all organisms. They facilitate rapid, highly selective water transport across the cell membrane [84]. These channels are so important that in 2003, the Nobel Prize in chemistry was awarded to Professor Peter Agre for his discovery of the aquaporin water channel. Aquaporin-embedded biomimetic membranes for nanofiltration have recently been conceptualized and fabricated, and can give an impressive water permeability and salt rejection [85][86]. These studies open up new possibilities of aquaporin embedded biomimetic membranes for water purification with advantages that include high throughput with less energy consumption. Aquaporin-based biomimetic NF membranes for suppliers of low-pressure household water purifiers and membranes for NASA to recycle astronaut urine in space has been developed on a commercial scale [87]. Although these artificial biomimetic systems hold tremendous promise by combining the functionality of biological channels with facile processability of synthetic materials, they are still in the nascent research phase with significant scale-up challenge. The drawback of the use of proteins in non-natural environments is that their life time may be limited by their stability and degradation. However, the major driver for development, such as biomimetic membrane technology, is that the conventional membrane technologies have reached their performance limit and new technologies with higher productivity and efficiency are essential.
Finally, the total global NF market is driven by its applications and demand from end user sectors, including water and wastewater treatment, food and beverages, chemical and petrochemicals, pharmaceutical and biomedical, textile and metalworking industry. Nanofiltration is highly adopted, growing technology and in water treatment NF are used not only for As removal but also water softening, color removal, as a barrier for removing various viruses and bacteria, industrial waste water treatment, water reuse, and even desalination. The increase in the use of chemical free water treatment procedures across various industries provide significant potential growth opportunities for the NF market in the future. The growth of the nanofiltration membrane market is expected to accelerate due to rapid urbanization and industrialization across emerging economies such as India and China; and increase in demand for water for domestic and industrial purposes, access to fresh and clean water. The global nanofiltration membrane market was valued at $643.22 million in 2017, and is projected to reach $954.65 million by 2025, growing at a compound annual growth rate (CAGR) of 5.4% from 2018 to 2025 [88]. However, high installation costs and lack of funds in the emerging economies may restrict the market growth.