1000/1000
Hot
Most Recent

Plants are sessile organisms that have a remarkable developmental plasticity, which ensures their optimal adaptation to environmental stresses. Plant cell totipotency is an extreme example of such plasticity, whereby somatic cells have the potential to form plants via direct shoot organogenesis or somatic embryogenesis in response to various exogenous and/or endogenous signals. Protoplasts provide one of the most suitable systems for investigating molecular mechanisms of the plant cell totipotency, because they are effectively single cell populations. The term protoplast originates from the ancient Greek word prōtóplastos, which means “first-formed”, and refers to a cell without a cell wall. Protoplasts represent a powerful tool to study the mechanisms that induce cell proliferation from individual, differentiated somatic cells, enabling successful reprogramming of plant cells when cultured in vitro.
The term protoplast originates from the ancient Greek word prōtóplastos, which means “first-formed”, and was proposed by Hanstein in 1880 to refer initially to a cell without a cell wall. One of the first successful protoplast isolations using enzymatic digestion was performed in 1965 from the parenchymatous placental tissue of immature tomato fruit by Gregory and Cocking [1]. Later, it was demonstrated that protoplasts can re-engage in cell division and form a callus, opening the possibility of plant regeneration (for review, see [2]). One of the first demonstrations of somatic embryogenesis was reported from carrot protoplasts in 1976 [3]. Thereafter, direct plant regeneration from protoplasts of mesophyll cells was achieved for a number of dicotyledonous species (for review, see [4]). The majority of investigations have been devoted to analysing gene expression during cell reprogramming, but detailed molecular genetics and epigenetic mechanisms of totipotency in angiosperms remain elusive.
A reliable source of quality protoplasts is necessary for studying cell reprogramming. There are two main criteria for selecting the tissue sources of protoplasts: (i) the type of organs and (ii) the type of the cells comprising the organs, i.e., cell competence. Protoplasts can be isolated from the leaves (mesophyll protoplasts), roots (root protoplasts) and callus (callus protoplasts). Protoplasts isolated from different organs have different biological profiles and therefore require different culture conditions. One of the most important factors is the status of the donor plant cells: differentiated with a low level of chromatin accessibility, e.g., mesophyll cells, or non-differentiated with a potentially active cell cycle and a high level of chromatin accessibility, e.g., callus and to some extent roots. Potential protoplast sources and their possible applications are listed in Table 1.
Table 1. Comparison of different angiosperm protoplast sources to study cell reprogramming (summarised from [4][5]).
| Leaf | Hypocotyl/Cotyledon | Root | Callus | |
|---|---|---|---|---|
| Homogeneity | yes | yes | no | yes |
| Reprogramming from differentiated to proliferating cells | yes | yes | no | no |
| Potential for totipotency | high for dicots, limited for monocots | high for young explants | high for dicots, limited for monocots | high for dicots and monocots |
This type of protoplast can be categorised into several subtypes, including cotyledon, hypocotyl and mesophyll protoplasts. Cotyledon protoplasts can be isolated from relatively young cotyledons before their cells undergo a terminal differentiation. The main advantage of this protoplast type is the relative uniformity of the starting material because all the cotyledons are the same age and have the potential to reach various levels of differentiation. The ability to determine to what extent the differentiation is reversible is a crucial point in an investigation of cell reprogramming. However, one has to consider the irregular ploidy of cells after the endocycles, and therefore, cotyledons can only be used as a protoplast source before entering the endocycles. The rapid process of differentiation in cotyledons is linked with the function of these organs in planta: the large cells with enhanced macromolecular production may require an increase in nuclear DNA contents, which fits well with cotyledon function as the carbohydrate source during early stages of seedling development. Later, the endopolypoid cells expand significantly and undergo terminal differentiation with a high level of chromatin condensation. Endopolyploidy in Arabidopsis may be directly linked with regulating cell size as a possible adaptation mechanism for growth of its relatively small cells [6]. However, it is technically challenging to isolate cotyledon protoplasts from Arabidopsis because the cotyledons are minute and difficult to separate from the very rapid formation of young leaves.
Hypocotyl protoplasts can be isolated from dark-grown seedlings and have similar advantages as cotyledon protoplasts, i.e., they provide a rather homogeneous and synchronised cell population [7]. However, the main disadvantage of both cotyledon and hypocotyl protoplast sources, especially from dark-grown hypocotyls, is the rapidly increasing cell ploidy level. For example, after five days, dark-grown Arabidopsis hypocotyls can have up to 30% of 16C cells [8]. A further disadvantage of this protoplast system is the large amount of seeds that are required and the time-consuming seed plating.
Because of the disadvantages detailed above, mesophyll protoplasts are the most commonly used among shoot-derived protoplasts. They can be isolated from differentiated mesophyll cells of different biological ages as well as from those at different stages [9][10]. The main advantage of mesophyll protoplasts that are isolated from dicotyledonous species, with the exception of in vitro grown Arabidopsis, is the possibility to obtain large amounts of relatively homogeneous cells. The developmental age of the leaves can also be determined [11], which is a critical step for explanting [12]. In dicotyledonous species, selecting the leaves for protoplast isolation is governed by the aim of the experiment, as leaves of different biological ages have differing capacities for cell de-differentiation. The ideal approach is to use only one fully expanded leaf as the protoplast source and to avoid cutting the main vein. Alternatively, two to three leaves that have the same position on the plant are also suitable. This is quite easy to achieve for dicots in which the mesophyll cells in a fully expanded leaf are of a similar age. By contrast, a gradient of differentiation is present in monocots due to their leaf growth, which starts from their base [13]. Mesophyll cells in the leaves of the grasses originate from meristematic cells, which are localised proximally to the meristem and have a rapid exit from the cell cycle. Therefore, only this fraction among isolated cells is capable of cell reprogramming. To date, no successful plant regeneration has been reported from monocotyledon leaf protoplasts.
Another source of shoot-derived protoplasts are guard cells [14], which are considerably more competent than mesophyll cells because of their higher chromatin accessibility and the absence of endocycles, which lead to a more regular chromatin organisation [15]. However, isolating guard cells is considerably more complicated technically than isolating protoplasts from other source cells.
The roots are another option for obtaining a population of isolated cells. Several protocols of root protoplast isolation are available for various species, e.g., various legumes [16][17][18], brassicas [19], Lycopersicum esculentum [20], Quercus rubra [21] and Pinus pinaster [22]. However, protoplast isolation from roots presents a significant technical challenge and does not ensure a homogeneous cell population. The different root zones require different enzyme combinations and different osmotic pressures [23]. This means that the digestion of a whole intact root produces a quite heterogeneous population of different cell types, which prevents a quantitative analysis of the process of cell development.
In conclusion, root protoplasts can be used for biotechnological applications such as fusing or transforming protoplasts but are not optimal for a systematic analysis of cell reprogramming due to their cell heterogeneity. However, the root protoplasts of Medicago sativa and some other members of the Fabaceae can be used as efficient models for analysing cell reprogramming during nodule formation [16]. The root protoplasts that are obtained from some monocots and dicots can be useful for patch-clump studies [24].
A callus comprises disorganised cell masses that are formed in response to hormone treatment and represents a portion of rapidly dividing cells [25]. An embryogenic callus that originates from these structures as immature embryos/inflorescences provides a homogeneous population of relatively non-differentiated cells. However, callus-originated protoplasts of monocotyledons can serve as a tool for studying the induction of cell totipotency. Although this type of protoplast is widely used to study grasses, in particular cereals, for biotechnological applications [26] it is not suitable for investigating the cell de-differentiation mechanisms because the cell cycles of the initial cells have already been activated.
Since mesophyll cells provide the most suitable and most popular starting material, we focus here on protoplasts derived from this tissue and describe all of the de-differentiation steps from the differentiated leaf cells to the totipotent cells and somatic embryos. Plant quality and isolation procedure determine the quality of isolated protoplasts, so we focus on these aspects below.
While the growth of donor plants does not need external plant growth regulator (PGR) supplementation, it does require proper internal hormonal balance, which is influenced by nutrient balance [27]. This is particularly important because particular nutrition can prevent a rapid endocycle and cell differentiation. It has been shown that the growth medium for donor plants of Arabidopsis is of significant importance for protoplast culture [28]. In a growth medium, all of the components serve as either a primary building material (N, P) or, as in the case of many micronutrients, contribute to this or other metabolic pathways. The most commonly used growth media often do not accommodate crucial nutrient functions in hormonal signalling because their components have been designed for rapid cell differentiation in the presence of certain phytohormone combinations. The optimal medium for plant growth should prevent nutritional stress, which, in turn, leads to a slowdown in the differentiation gradients in leaf cells and extends the competence window.
The concept that cell reprogramming depends on the cell developmental stage first came from an investigation of plant regeneration from the leaf tissue of barley. In this system, the cells undergo very rapid differentiation and only the segments close to the meristem are able to re-enter the cell division cycle [29]. Similarly, immature embryos of wheat also have a very strict competence window [30][31], which occurs when the scutellar tissue (the source of the embryogenic callus) remains active and visually appears to be semi-transparent. This is logical because the process of tissue development from initial cells undergoes several steps but only the early ones are reversible. This is true not only for monocotyledons, but also for dicotyledonous species in which the ability of protoplast regeneration is linked with the biological age of the explants [12]. There are two main reasons for this: (i) chromatin condensation, which can be reversible only under certain conditions and (ii) an irregular ploidy level in differentiated cells, which is irreversible. Therefore, determining the ploidy level and chromatin accessibility in isolated protoplasts is necessary before their culture.
Isolating cells from their native tissue and organ environment can potentially induce apoptosis [32]. This means that the procedure of protoplast isolation is the most important step for ensuring that optimal starting material is obtained. During this procedure, three criteria must be adhered to: (i) the homogeneity of the starting material (only organs of the same biological age can be used, i.e., a single leaf or only the cotyledons); (ii) regular ploidy level of isolated protoplasts. Therefore, ploidy level should be determined using flow cytometry; (iii) the damaging effect of the isolation procedure must be minimised by a gentle cutting, using cellulolytic enzymes, limited centrifugation steps, etc. All of these precautions are particularly important for mesophyll cells, which, once they exit the cell cycle, have condensed chromatin [33], a low level of the “cytoplasmic” antioxidant system and are starting the apoptotic pathway, and whose only function is to supply carbohydrates to developing tissue. The isolation procedure can also induce further chromatin condensation and the apoptotic pathway [34]. This condensation is accompanied by a reduction in the scavenging capacity of reactive oxygen species (ROS) [35], which leads to an increased ROS accumulation [36][37]. Recently, the presence of chromatin condensation has also been demonstrated during protoplast isolation followed by subsequent cultivation in a buffer without PGRs [38].
There are several options for reducing the negative aspects of the protoplast isolation procedure. For example, almost all of the commercially available cellulolytic enzymes are rather crude extracts that contain different proteases/nucleases. Therefore, their removal is crucial for preventing isolated cell degradation, which in turn improves protoplast quality. This can be done either by incubating a crude enzyme solution at 55 °C for 10 min [39] or by decreasing pH to 3.5 for a short time. The ionic composition of a digestion solution is another important consideration: for example, adding certain ions (cell and protoplast washing solution) [40] or antioxidants, such as ascorbic acid, to the enzyme mixture has a significant positive effect on the quality of isolated protoplasts. A good example of such a strategy is the buffer composition for preventing cytosolic acidification that was recently proposed for Arabidopsis [41].
Based on the stages of cell differentiation from the SAM to the mature leaf, we can distinguish three stages in the de-differentiation of the mesophyll protoplast [42][43], which are in reverse to the differentiation stages and which include the induction of the reprogramming process, the epigenetic remodelling of the chromatin and the induction of totipotency (Figure 1).
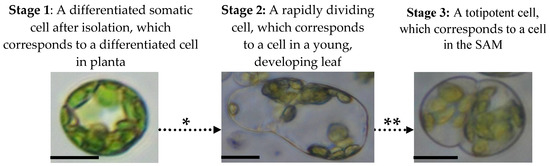
Figure 1. Mesophyll protoplast reprogramming to totipotency. There are three stages that mesophyll protoplasts follow during the activation of their cell division and transition to totipotency which are accompanied by various epigenetic, physiological and molecular processes. The following events occur during mesophyll protoplast reprogramming and cause dynamic changes in chromatin accessibility, hormonal responses and reactivation of the cell cycle: * Chromatin relaxation caused by specific histone and DNA chemical modifications, hormonal/ROS signalling (activation of auxin response and ROS generation/scavenging) and changes in the cell cycle gene expression. ** High-auxin environment leads to protein storage vacuole transition, histone hyperacetylation and cell cycle extension. Photomicrographs show protoplasts of Medicago sativa. All bars: 10 µm.
Changes in the epigenetic chromatin status of differentiated cells is the first step in their conversion to the proliferation pathway [43][44]. There is a dearth of comprehensive studies on the epigenetic changes during mesophyll protoplast cultivation to date. However, according to data that are available for several dicotyledonous species, it is clear that reactivation of the cell cycle is accompanied by increased chromatin relaxation, decreased DNA methylation and changes in histone structure (Table 2).
Table 2. Studies on the epigenetic status of mesophyll protoplasts during in vitro culture.
| Species | Approach | Process | References |
|---|---|---|---|
| Nicotiana tabacum | fluorescence-activated cell sorter (FACS); gel electrophoresis of DNA after micrococcal nuclease (MNase) digestion | chromatin condensation/decondensation | [34] |
| Cucumis sativus | FACS; fluorescence in situ hybridisation | chromocentre and repeat reassembly | [45] |
| Medicago sativa | flow cytometry; nucleus morphology |
chromatin relaxation; DNA stainability | [9][43][46] |
| Nicotiana tabacum | nucleus morphology; gene expression | histone H3 modifications; redistribution of HP1; activation of the E2F transcription factor genes | [47] |
| Brassica oleracea; Cucumis sativus | quantification of methylated and hydroxymethylated DNA | temporal changes in the amount of 5-mC and 5-hmC | [48] |
Besides these epigenetic changes, mesophyll protoplast reprogramming is accompanied by significant modifications in cell structure and physiology including changes in various aspects of its ultrastructure, cytoskeleton and ROS-level vacuolar function.
The first analyses of mesophyll protoplast physiology were performed in the 1970s. These early investigations were reviewed in detail by Galun [49]. Among the physiological parameters that were analysed, oxidative stress responses and changes in the cell ultrastructure were found to be the main hallmarks associated with cell reprogramming. Differentiated mesophyll cells contain a large central lytic vacuole that is characterised by low pH. This vacuole governs the distribution of cytoplasm and organelles to the cell periphery and prevents cell proliferation. During the cultivation period, the vacuole becomes more alkaline [50] and numerous transvacuolar strands arise [51]. Finally, the vacuole divides into several smaller vacuoles, which become protein storage sites during the pro-embryogenic cell divisions [35][46]. The structure of the cytoplasm also changes significantly during the conversion of mesophyll cells into proliferating cells, which is accompanied by changes in the ion composition and total soluble protein profiles. Up to 70% of the soluble protein fraction in mesophyll cells is RuBisCO (~70 kDa), while in proliferating cells, the amount of this enzyme contributes only 10% along with an increasing amount of cytoplasmic proteins.
ROS/redox balance is another key parameter in the cell reprogramming process. Several studies have suggested that the differences in the ROS level between regenerating and non-regenerating protoplasts are the main causes of cell recalcitrance [52][53][54]. Significant changes in ROS generation and scavenging, the antioxidant level, cell structure and vacuolar pH have also been reported [43][50].
Hormones are a key signal for stimulating cell reprogramming. Among the hormones, auxins are not only required for cell cycle activation [54] and essential for the induction of chromatin relaxation and DNA replication [43] but are also indispensable for somatic embryogenesis in general [55]. Cytokinins are key hormones that are involved in the process of cytokinesis. The complex interaction between these two hormone groups occurs during somatic embryogenesis [56]. Other hormones or PGRs do not seem to be so critical for cell reprogramming but may act by modulating the effects of auxins. For example, the application of brassinolides or salicylic acid can upregulate the auxin signalling in Arabidopsis mesophyll protoplasts. In their study of the nonphototropic hypocotyl4-1 mutant which is null for the AUXIN RESPONSE FACTOR7 (ARF7) transcriptional activator, Wang, et al. [57] clearly demonstrated the reduced expression of integrated auxin-responsive reporter genes and endogenous genes in Arabidopsis leaf mesophyll protoplasts. Since the mutants of other ARFs did not show any altered expression in reporter or endogenous auxin response genes, it was likely that ARF7 played a major role in regulating auxin effects in leaf mesophyll cells. It further points out that while interactions between hormones and/or PGRs have been investigated in detail at the whole plant level, similar analyses for protoplasts are still lacking and are noteworthy subjects for future investigations.
Stress in combination with auxin is another key factor that is responsible for executing the cell totipotency programme. However, one should distinguish between a stress in response to stress-induced agents, and a combination of stress and hormonal signalling. For example, it has been shown that the application of auxins in combination with stress-inducing agents are required for successful cell reprogramming in M. sativa and Arabidopsis and do not lead to actual oxidative stress as determined by H2O2 level [35][58]. On the other hand, the inhibition of ROS generation and increasing ROS scavenging halt protoplast reprogramming. Low molecular weight antioxidants such as ascorbate and glutathione are considered to be ROS scavengers. Interestingly, while ascorbic acid acts as the main ROS scavenger by inhibiting cell proliferation in both M. sativa and N. tabacum, glutathione seems to have an opposite effect [44][59]. Nitric oxide seems to have a similar effect [60], which in turn leads to changes in the chromatin architecture [61].
De-differentiated cells are different from each other. There are two types of cells in planta: the slow proliferating cells in the SAM and RAM stem cell niches that have an unspecified fate, and the rapidly proliferating ones in the developing organs after cell fate has already been established [62]. Rapidly proliferating cells in roots constitute a population after cell fate has already been determined and therefore cannot give rise to all of the cell types [63]. Only a small portion of stem cells in the SAM and RAM in planta can be considered to be totipotent and these cells are characterised by specific features such as small nuclei, hyperacetylated histones, an extended duration of G1 phase and the presence of protein storage vacuoles. In the majority of cases of reprogramming mesophyll protoplasts, the rapidly proliferating cells can only form a callus, i.e., not totipotent cells. However, it is possible to convert them into SAM-like cells that are able to develop directly into somatic embryos and shoots. From this point of view, the second step in cell de-differentiation is the “creation” of totipotent cells that are capable of being converted to shoots through somatic embryogenesis or organogenesis [64].
Only a few cells in whole plants have the features of stem-like cells. Their low abundance makes it difficult to study molecular features of these cells, but culturing protoplasts can circumvent this problem. However, it should be borne in mind that stem-like cells in planta exist in a specific local environment, conditions that need to be reproduced as closely as possible in mesophyll protoplast cultures. By using this approach, it is possible to induce totipotent cells in vitro that have the features of stem-like cells and thus have the potential to generate all cell types. This enables investigation using the standard molecular biology methods of potentially all of the factors that are responsible for stem cell induction in a similar manner to the study of Physcomitrella patens [65]. For example, using this approach, Sakakibara, et al. [66] showed differences in gene expression in this model moss and the key role of WOX genes, which serve as epigenetic regulators. In vascular plants, a similar system has been described for M. sativa mesophyll protoplasts in which after the application of stress-inducing factors, somatic cells were converted into totipotent cells that had typical stem cell features [35][44][50]. The physiological and genetic mechanisms of this transition include changes in hormonal signalling, cell cycle duration, cell morphology, ROS scavenging activities, content of ascorbate/glutathione, chromatin organisation and gene expression [35][67].