1000/1000
Hot
Most Recent

Non-mechanical hybrid hydrogen compressors consists of a first electrochemical compression stage followed by a second one based on the adsorption-desorption of hydrogen on microporous materials. They allow compressing hydrogen up to 70 MPa. Non-mechanical hybrid hydrogen compressors can be a valid alternative to the mechanical compressors.
Non-mechanical hydrogen compressors have proven to be a valid alternative to mechanical compressors. Among these, electrochemical compressors allow isothermal, and therefore highly efficient, compression of hydrogen. On the other hand, adsorption-desorption compressors allow hydrogen to be compressed through cooling/heating cycles using highly microporous materials as hydrogen adsorbents. A non-mechanical hybrid hydrogen compressor, consisting of a first electrochemical stage followed by a second stage driven by adsorption-desorption of hydrogen on activated carbons, allows hydrogen to be produced at 70 MPa, a value currently required for the development of hydrogen automotive applications. This system has several advantages over mechanical compressors, such as the absence of moving parts and high compactness. Its use in decentralized hydrogen facilities, such as hydrogen refueling stations, can be considered.
Irreversible environmental issues will be inevitable if the global energy scenario continues to rely on fossil fuels for a long time. CO2 emissions have increased significantly in recent years, reaching 34 Mt per year, 40% higher than in 2010 [1]. The main consequence is the intensification of the greenhouse effect on our planet, and with it the increase in its average temperature. Indeed, Earth’s average temperature has increased by just over 1 K since 1880. Two thirds of global warming has occurred since 1975, at a rate of about 0.15–0.20 K per decade [2]. At this rate, global warming will be detrimental to ecosystems and human health, as infectious diseases will emerge due to the warmer and wetter environment.
For all the aforementioned reasons, the search for energy sources that could be both environmentally friendly and limitless is currently in great demand. The use of hydrogen as an energy vector fits perfectly into this framework, especially if it is produced from renewable sources. Indeed, hydrogen is a clean energy vector, and it is advocated as a serious candidate to replace fossil fuels, especially in the transport sector. Stationary hydrogen storage can also be envisaged as a guarantee of energy supply in the event of power grid failure or fluctuations in wind and solar energy. Indeed, the intermittency of renewable energy sources implies their storage in efficient and reactive storage systems, giving rise to the concept of smart grids. In this framework, power-to-hydrogen systems use expanding and inexhaustible renewable energy resources to power electrolyzers, producing hydrogen from water [3][4], thus reducing the load on the electricity grid and the risk of power outages [5]. Thus, electricity can be used to produce hydrogen by water electrolysis, and electrical energy can be produced by using hydrogen in fuel cell systems.
Since hydrogen is widely used in industry for the production of ammonia and the hydrogenation of petroleum products, the hydrogen sector, which includes production in decentralized facilities, storage and distribution, is already mature. However, the potential benefits of hydrogen as a fuel can be realized once storage methods are optimized and an efficient and safe distribution infrastructure is in place. In this context, the storage of hydrogen requires its compression. Mechanical compressors (piston, diaphragm, linear, and ionic liquid compressors), which are universally used for the compression of all gases, are not very suitable for the specific case of hydrogen. Research on new compression technologies, such as non-mechanical hydrogen compressors (metal hydrides, electrochemical, and adsorption-desorption compressors) is thus highly demanded, particularly with regard to the development of decentralized infrastructure for the production and use of hydrogen in situ.
Non-mechanical hydrogen compressors have several advantages over mechanical compressors, including: (i) no moving components; (ii) quiet operation; (iii) high reliability and safety; and (iv) structural simplicity and greater compactness.
Of all non-mechanical hydrogen compressors, metal hydride compressors have attracted significant attention in recent years. They are thermally driven compressors because they use the properties of hydride-forming metals, alloys, or intermetallic compounds to absorb and desorb hydrogen simply by heat and mass transfer in the reaction system. Hydrogen absorption occurs at low temperature and lasts until the equilibrium pressure is equal to the feed pressure. When the metal hydride is heated, the hydrogen is desorbed and released at a higher pressure [6]. Metal hydride compressors are thoroughly described elsewhere [7]. No moving parts are present in a metal hydride compressor, which prevents the use of lubricating oils as needed in the case of mechanical compressors. However, this technology is limited both by the performance of the hydrides used and by heat management. Indeed, a multi-stage configuration is required to allow hydrogen compression up to 70 MPa [8]. This means that different types of metal hydrides have to be used in series, so that the desorption pressure of the first stage at high temperature can be slightly higher than the absorption pressure of the next stage at low temperature. In this way, the hydrogen is progressively compressed. Despite this, high desorption temperatures must be used in order to achieve high discharge pressures. To date, the average desorption temperature in metal hydride compressors is typically about 573 K, which significantly reduces efficiency by up to 10% [7]. Nevertheless, if the discharge pressure of a metal hydride compressor is sufficiently low, its cost may be lower than that of a mechanical hydrogen compressor operating at the same compression ratio [9].
Based on the same principles as proton-exchange membrane fuel cells (PEMFCs), the electrochemical hydrogen compressor (EHC) has proven to be the most appropriate choice when hydrogen compression by a convenient, compact, cheap, and high-efficiency system is required [10]. Low-pressure hydrogen is fed to the anode of an electrochemical cell consisting of two electrodes, a polymer membrane, and gas diffusion layers. Here, the hydrogen is oxidized (Figure 1), thus splitting into protons and electrons, while electrical energy is supplied to the system. While the electrons follow the external electric circuit driven by a power supply, the protons pass through the polymer membrane to the cathode, where the hydrogen reduction reaction takes place. Hence, molecular hydrogen is produced there. The use of a backpressure regulator allows a flow of hydrogen at the desired discharge pressure. It is important to highlight that, unlike PEMFCs, the cathode of an EHC is blocked, i.e., no air is introduced. EHC requires very efficient core materials [11]. Nafion® is generally used as a membrane for EHCs [12]. Indeed, Nafion® offers high proton conductivity (0.13 S cm−1 at 348 K and 100% relative humidity), durability above 60,000 h, and high chemical stability [13]. Membrane-electrodes assemblies (MEA) are used to speed up the electrochemical process, in which metal nanoparticles, especially platinum, are dispersed in a solid electrolyte matrix in a similar way as PEMFCs, because of their excellent catalytic properties [14].
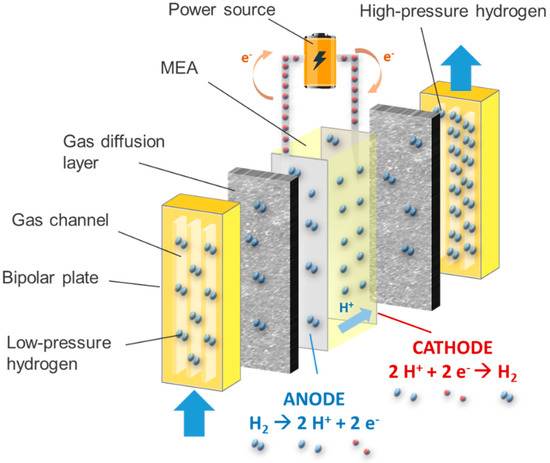
Figure 1. Scheme of the components and reactions taking place in an electrochemical hydrogen compressor.
The compression mechanism described above is purely electrochemical, so that no moving unit is needed to drive it. This translates into a very high efficiency, up to 60% [15]. Furthermore, the EHC provides isothermal compression of hydrogen, which requires a lower energy demand compared to a polytropic or adiabatic process [16], and very high discharge pressures can be reached, even up to 100 MPa [17]. Despite all of these advantages, the efficiency of an EHC decreases considerably as the discharge pressure increases. Indeed, the permeation of molecular hydrogen through the PEM from cathode to anode increases linearly with the pressure difference over the EHC [18], reducing the amount of high-pressure hydrogen at the outlet of the EHC. This phenomenon is also known as “back-diffusion”. For this reason, the use of EHC was found to be more appropriate for low-pressure applications, such as power-to-gas or as a pump for hydrogen recirculation in fuel cell vehicles [19], as well as in high-pressure hybrid systems where the EHC performs a first pre-compression stage [20][21]. In addition, the EHC can also function as a purifying device, which is an important advantage when hydrogen is mixed with other gases, e.g., in H2-CH4 hythane mixtures [22]. Compared to other conventional means of hydrogen purification and compression, the EHC combines low energy cost, high H2 recovery and purity, low maintenance, low cost, and low operating temperature [23].
The adsorption of hydrogen on nanotextured materials, thus having both a high specific surface area and microporosity, has been studied in depth in the context of hydrogen storage in solids, as shown in the previous sections. In addition, hydrogen physisorption on microporous materials can be exploited to drive hydrogen compression, which represents a new and innovative way of compressing hydrogen in a non-mechanical way, and is therefore worth exploring.
An adsorption–desorption compressor is a thermally driven compressor, just like metal hydride hydrogen compressors. Therefore, hydrogen compression comes from thermal cycles consisting of progressive cooling and heating stages. Hydrogen adsorption is initially carried out at cryogenic temperatures. Indeed, the density of adsorbed hydrogen increases considerably as the temperature of the system is lowered. It is generally assumed that the density of the adsorbed hydrogen can be approximated to the density of liquid hydrogen [24][25]. Some authors have even observed a behavior similar to that of solid hydrogen in the adsorbed phase [26]. Hence, hydrogen adsorption is generally carried out at 77 K, i.e., at the temperature of liquid nitrogen, which is easy to achieve from an industrial point of view. Under these conditions, the density of the adsorbed hydrogen is thus equal to 70.8 g L−1.
Hydrogen compression comes from the desorption of the pre-adsorbed amount of hydrogen. This is because hydrogen passes from the adsorbed phase, which is denser, to the bulk phase in a confined tank volume when the temperature rises. This can be done by removing the Dewar vessel filled with liquid nitrogen, where the compression tank is initially placed to drive the adsorption, thus leaving the tank at room temperature. Alternatively, a cooling system can be designed and placed inside the tank, in contact with the microporous adsorbent material, to manage better temperature gradients (Figure 2). In addition, microporous materials with high thermal conductivity should be used in order to increase the kinetics of adsorption and desorption. For instance, activated carbons, which have been shown to be well suited for hydrogen adsorption with their many advantages [27], have an average thermal conductivity of about 0.2 W m−1 K−1 [28], which may decrease the efficiency of the adsorption–desorption compressor. Nevertheless, the use of composite adsorbents, such as mixed powders of flexible graphite and activated carbon, can increase the effective thermal conductivity of the porous bed [29].
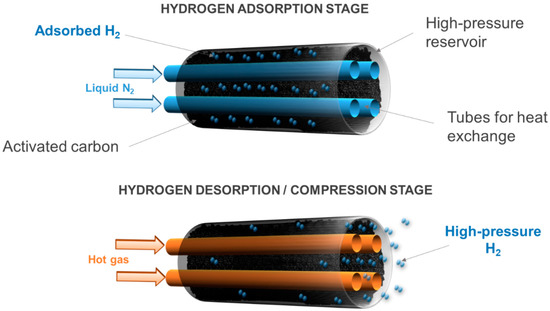
Figure 2. Operating principles of an adsorption–desorption compressor.
The adsorption–desorption compressor has all of the common advantages of non-mechanical compressors. Indeed, even if this technology is still too new to allow an accurate assessment of its performance and costs, the absence of moving parts undoubtedly contributes significantly to the reduction of installation and maintenance costs compared to mechanical compressors.
Reducing the cost of hydrogen storage is crucial for the development of automotive hydrogen applications, such as fuel cell vehicles. In fact, the storage, transportation, and distribution stages cause significant increases in the price of hydrogen at the pump, which is currently at USD 8–10 kg−1.
High-pressure hydrogen storage has been proven to be the most suitable method for storing hydrogen in decentralized facilities, i.e., at hydrogen refueling stations, compared to liquid-phase storage and storage in absorbed form in solid materials. Nevertheless, mechanical compressors, which are the most widely used technology for compressing hydrogen today, are responsible for more than 50% of CAPEX, 20% of OPEX, and about 30% of the total energy consumption of a hydrogen refueling station. Furthermore, mechanical compressors have several disadvantages, such as the presence of many moving parts, hydrogen embrittlement, high consumption of energy, high structural complexity, and difficult heat management.
Non-mechanical compressors, such as metal hydride, electrochemical, and adsorption–desorption compressors, may be a suitable alternative to replace mechanical compressors in decentralized facilities. Indeed, they have several advantages, such as the absence of moving parts that contributes significantly to the reduction of installation and maintenance costs compared to mechanical compressors. Metal hydride compressors ensure both safe storage and compression of hydrogen. As they require heat exchange, they are also known as thermally driven compressors. The search for appropriate alloys is essential for the development of such technology, as it requires both low desorption temperatures and high pressures.
Electrochemical compressors are based on the use of selective polymeric membranes, such as Nafion®, to compress hydrogen gas, and have been proven to provide the highest level of compression efficiency (up to 60%) when the discharge pressure is not too high. Indeed, the efficiency of such devices is affected by the hydrogen back-diffusion. Electrochemical compressors have been found to offer good performances when equipped with a thin membrane at 1 A cm−2.
Adsorption–desorption compressors rely on the ability of hydrogen to bind weakly to the surface of highly porous solids, such as carbon materials or metal-organic frameworks. Like metal hydride compressors, adsorption–desorption compressors are also thermally driven. Nevertheless, operation at a cryogenic temperature, down to 77 K, is required to enhance hydrogen uptakes. Such devices are able to compress hydrogen up to 70 MPa in a single step.
Hybrid configurations, consisting of: (i) a first electrochemical stage up to 4–8 MPa; and (ii) a second stage based on cyclic adsorption–desorption on carbon materials, make it possible to reach 70 MPa in a compact and quiet device. This could represent a promising alternative to mechanical hydrogen compressors in the framework of decentralized facilities, such as hydrogen refueling stations.