1000/1000
Hot
Most Recent

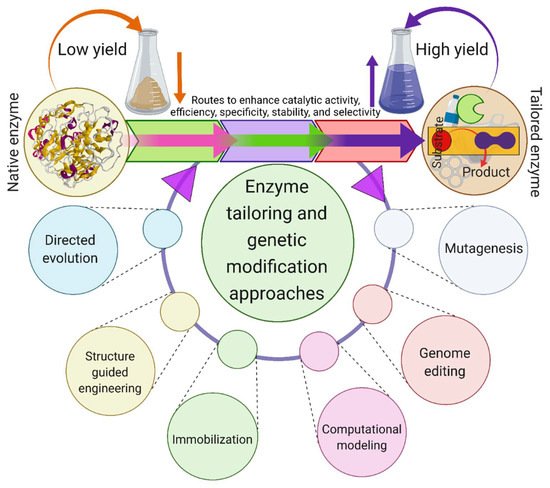
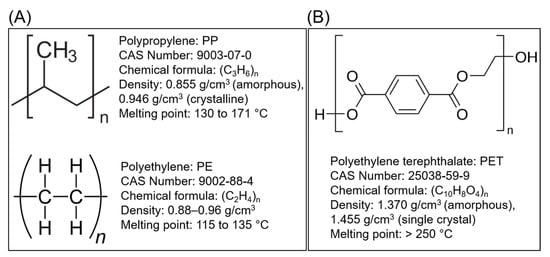
The ubiquitous persistence of plastic waste in diverse forms and different environmental matrices is one of the main challenges that modern societies are facing at present. The exponential utilization and recalcitrance of synthetic plastics, including polyethylene terephthalate (PET), results in their extensive accumulation, which is a significant threat to the ecosystem. The growing amount of plastic waste ending up in landfills and oceans is alarming due to its possible adverse effects on biota. Thus, there is an urgent need to mitigate plastic waste to tackle the environmental crisis of plastic pollution. With regards to PET, there is a plethora of literature on the transportation route, ingestion, environmental fate, amount, and the adverse ecological and human health effects. Several studies have described the deployment of various microbial enzymes with much focus on bacterial-enzyme mediated removal and remediation of PET. However, there is a lack of consolidated studies on the exploitation of fungal enzymes for PET degradation. Herein, an effort has been made to cover this literature gap by spotlighting the fungi and their unique enzymes, e.g., esterases, lipases, and cutinases. These fungal enzymes have emerged as candidates for the development of biocatalytic PET degradation processes. The first half of this review is focused on fungal biocatalysts involved in the degradation of PET. The latter half explains three main aspects: (1) catalytic mechanism of PET hydrolysis in the presence of cutinases as a model fungal enzyme, (2) limitations hindering enzymatic PET biodegradation, and (3) strategies for enhancement of enzymatic PET biodegradation.
| PET Packaging Products | Global Consumption in 2020 (Million Tonnes) |
|---|---|
| Water Bottles | 7.02 |
| Carbonated soft drink (CSD) bottles (e.g., Coca Cola, beers) | 7.02 |
| Other drinks (e.g., juices, milk) | 4.86 |
| Other bottles/containers in form of films and sheets | 3.78 |
| Food containers | 2.43 |
| Containers for non-food consumer products (e.g., cosmetics) | 1.62 |