Radiation exists in two main forms: Electromagnetic (EM) radiation in the form of alternating electric and magnetic waves that propagate energy, and particle radiation consisting of accelerated particles such as electrons and protons. EM radiation can be broadly categorised as non-ionising and ionising. Both types may be encountered clinically or environmentally, with exposure having potentially positive or negative effects on tissues and organisms (
Table 1). In the case of non-ionising radiation, exposure of skin to ultraviolet radiation (UVR), for example, may be beneficial, as a consequence of vitamin D production
[1], or detrimental, due photoageing
[2] and/or photocarcinogenesis
[3]. UVR is considered non-ionising as it is, in general, not sufficiently energetic to remove electrons from biomolecules. In contrast, energetic, ionising electromagnetic radiation (X-rays and gamma rays) can remove electrons. The undoubted importance of controlled exposure to ionising EM radiation in medical diagnostic imaging
[4] and radiotherapy
[5][6] must be balanced against side effects such as secondary cancers or tissue fibrosis
[7][8]. Other forms of radiation, which rely on charged particles (e.g., α, β, protons), can also interact with biological systems and are clinically important (such as in proton therapy and in cosmic radiation exposure for space exploration), but being non-electromagnetic, they lie outside the scope of this review. The reader is referred to an excellent review by Helm et al.
[9].
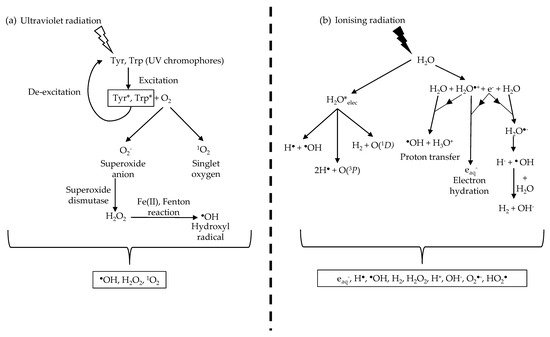
Most investigations into the detrimental side effects of radiation on biological tissues have largely focused on cellular damage, and in particular, the sensitivity of DNA
[27][28]. Whilst acute high radiation exposure may kill cells, it has become increasingly clear that lower doses may have sub-lethal effects that are complex, difficult to eliminate and delayed (persisting over long periods of time)
[2][7][29][30]. Crucially, to understand the consequences of radiation exposure and hence to potentially prevent or reverse the damage, it is necessary to characterise the interactions of radiation with not only cells but also with their complex and dynamic extracellular environment. This review considers the consequences and causative mechanisms that drive electromagnetic radiation damage in biological tissues and in the extracellular matrix (ECM) in particular. Two clinical models of interest are discussed: skin exposed to UVR in sunlight and breast tissues exposed to diagnostic and therapeutic X-rays.
Electromagnetic Radiation
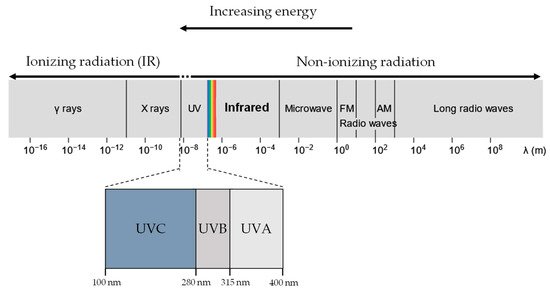
UVR and X-rays/Gamma rays, both being part of the EM radiation spectrum (
Figure 1), differ only in wavelength, frequency and energy. When a molecule absorbs EM radiation, it undergoes one of three possible transitions: electronic, vibrational, or rotational
[31]. In general, electronic transitions require the largest amount of energy, followed by vibrational then rotational
[32].
Figure 1. UVR, X-rays and gamma rays all lie in the electromagnetic spectrum. UVR (UV-A and UV-B) lie at a slightly higher energy range compared to visible light and are generally considered non-ionising. In contrast, X-rays and gamma rays have much higher energy than UVR and are considered ionising radiation.
Ionising radiation is often more energetic than non-ionising radiation and, as a result, is more likely to induce electronic transitions of atoms and molecules. In electronic excitation, an electron absorbing the radiation transits into a higher electronic state, becoming less bounded to the nucleus and therefore more reactive
[33]. If the radiation has sufficient energy, the electron can escape the coulomb attraction of the nucleus, and the molecule is ionised. In contrast, molecules undergoing rotational or vibrational transitions (generally caused by non-ionising UVR exposure) experience minimal changes in the stability of the electron-nucleus attraction, resulting in negligible chemical effects. Therefore, exposure to ionising and non-ionising radiation results in significantly distinct biological molecular effects.