L. gmelinii is widespread in Eastern Europe, South-Western Siberia, China, and Central Asia. It has industrial resources on the whole territory of Kazakhstan, primarily on saline lands, and has been used in traditional herbal medicine in Central Asia for hundreds of years. These plants are characterized by rapid growth and high yield, so their reserves in nature can be kept at their original level if the guidelines of good practices for collection and preparation of medicinal plant raw materials are followed. These plants can also be cultivated.
1. Introduction
Oxidative stress is induced by an imbalance of the redox system due to the overproduction of reactive oxygen species (ROS) and/or the disturbance of the antioxidant systems. The brain is an organ that is especially susceptible to the effects of ROS due to its high oxygen demand, increased levels of polyunsaturated fatty acids in neuronal cells, and relatively low levels of antioxidant enzymes
[1]. ROS damage lipids, proteins, DNA, RNA, and alter signaling pathways leading to necrotic and apoptotic neuronal death
[2]. Additionally, increased ROS stimulates cerebral endothelial cells and astrocytes to secrete inflammatory cytokines and chemokines, and causes the upregulation of adhesion molecules in the cerebral vasculature and the recruitment of peripheral leukocytes
[3][4]. The deployment of immune cells is primarily designed to tackle pathogens, and their activation during the sterile immune response further destroys nerve tissue
[5]. Previous studies have demonstrated that oxidative stress is one of the leading pathophysiological reactions participating in the development and progression of neurodegenerative diseases such as Alzheimer’s disease and Parkinson’s disease
[1], and it also plays a central role in ischemic brain injury
[5]. Thus, agents targeting ROS production, activation of pro-inflammatory cytokines, and expression of adhesion molecules have therapeutic value in brain disorder therapy.
As oxidative stress is one of the universal mechanisms of different pathological conditions in the brain, antioxidants have been considered as therapeutics and preventive measures to inhibit neuroinflammation and detrimental impacts of ROS
[6]. There are lots of compounds that have prominent antioxidant and anti-inflammatory potential. Compounds such as phenolic acids, proanthocyanidins, and flavonoids are attracting great interest because of their ability to scavenge free radicals and inhibit oxidative damage. Nowadays, plant polyphenols occupy a unique place in medicine as they are potentially capable of reducing the risk of many diseases and might be used in the treatment of diabetes, cardiovascular disorders, atherosclerosis, neurodegenerative disorders, and inflammation.
Polyphenols are natural organic molecules consisting of a huge number of phenol structural units. They are commonly presented in vegetables, fruits, grains, bark, roots, tea, and wine. Plant polyphenols possess many unique physical, chemical, and biological (metabolic, toxic, therapeutic, etc.) properties directly dependent on the number and characteristics of phenol units and their structure. Most polyphenols are generally known to possess potent antioxidant, anti-inflammatory, and anti-apoptotic properties, and their neuroprotective effects have been demonstrated in several in vitro and in vivo studies
[7][8][9][10]. For example, magnolia polyphenols have been shown to attenuate oxidative and inflammatory responses in neurons and microglia cells
[8]. Green tea polyphenols have also been proven to exhibit multiple neuroprotective actions
[11][12]. However, the health effects of polyphenols depend on the chemical structure (e.g., glycosylation, esterification, and polymerization) and bioavailability. Bioavailability appears to differ greatly between the various polyphenols, and the most abundant polyphenols in our diet are not those that have the best bioavailability profile
[13]. Thus, new plant sources rich with bioavailable polyphenols are still in demand.
We have previously reported that the plant substance extracted from roots of
Limonium gmelinii (
Plumbaginaceae) possesses significant anti-oxidative and hepatoprotective activities, exceeding those of single flavonoids
[14]. This plant is a rich source of flavonols and their glycosides, phenolic acids, monomeric, dimeric, and oligomeric forms of flavan-3-ols; it also contains other biologically active compounds, including all known 20 natural α-amino acids, vitamins C, E, and ß-carotene.
L. gmelinii is widespread in Eastern Europe, South-Western Siberia, China, and Central Asia
[15]. It has industrial resources on the whole territory of Kazakhstan, primarily on saline lands, and has been used in traditional herbal medicine in Central Asia for hundreds of years. These plants are characterized by rapid growth and high yield, so their reserves in nature can be kept at their original level if the guidelines of good practices for collection and preparation of medicinal plant raw materials are followed. These plants can also be cultivated. In the present study, we have assessed antioxidant and anti-inflammatory properties of plant extract from
Limonium gmelinii in neurons, astrocytes, and cerebral endothelial cells (CECs) in vitro, and evaluated its therapeutic potential on animal models of ischemic brain injury. Our choice of cells was dictated by the fact that, as mentioned before, neurons are highly susceptible to oxidative stress
[1]. In turn, increasing evidence exists, demonstrating that both astrocytes and cerebral endothelial cells are intensively involved in different aspects of CNS homeostasis including oxidative stress regulation, and the redox level in CNS can also affect astrocytes and CECs in morphology and function
[3][4].
2. Chemical Characterization of the Plant Substance
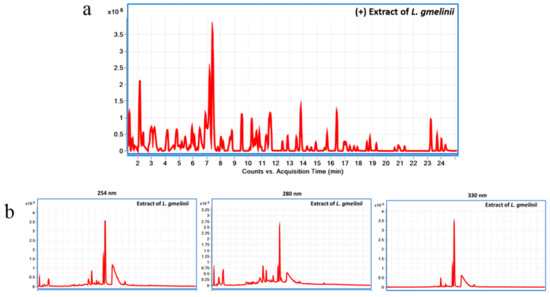
LC-MS chromatogram of
L. gmelinii extract in (+)ESI mode using LC-QToF is presented in
Figure 1a, and LC-UV chromatogram of the extract at 254, 280, and 330 nm using LC-DAD is presented in
Figure 1b. The chemical composition of the plant substance is presented in the supplementary
Table S1. In short, root extract of
Limonium gmelinii contains a unique set of polyphenols in the form of phenolic acids (gallic and ellagic), flavonols, mainly aglycone myricetin and its glycoside forms, condensed tannins, the latter being represented by dimeric and oligomeric forms of flavan-3-ols. In the chemical study of the substance flavonols (myricetin, quercetin, kaempferol, and the new 3,4,5,3′,4′,6′-hexahydroxyoxyflavone), their glycosides (myricitrin, rutin, 3-β-galactosylquercetin, 3-β-galactosylmyricetin, new 3-α-galactopyranoside myricetin and 3-
O-α-
l-(2″-galloyl)-arabinopyranoside myricetin), pyrogallol are identified. The main monomeric flavan is (-)–epigallocatechin gallate. The new forms of flavan-3-ols, namely the 3,5,7,3′,4′,6′-hexahydroxyflavan, (-)–epigallocatechin-(4β→8)-(-)-3,5,7,3′,4′,6′–hexahydroxyflavan, and the (+)–gallocatechine–(4α→8)–[(-)–epigallocatechin]5–(4β→8)–(-)–epigallocatechin gallate, was found in the
L. gmelinii root extract
[16]. The quantitative content of tannins in the substance was 43.2%, flavonoids—15.23%, organic acids—6.36%, carbohydrates—5.44%
[17]. Safety study of the substance conducted at the Test Center of the National Center of drugs, medical products, and medical equipment examination of the Ministry of Healthcare of the Republic of Kazakhstan has shown that it has no toxic effect on the animal organism and thus may be attributed to the group of non-toxic substances.
Figure 1. Chemical characterization of L. gmelinii root extract using Liquid chromatography diode array detector-quadrupole time-of-flight mass spectrometry (LC-DAD-QToF). LC-MS chromatogram of L. gmelinii extract in (+)ESI mode using LC-QToF (a); LC-UV chromatogram of L. gmelinii extract at 254, 280, and 330 nm using LC-DAD (b).
3. L. gmelinii Extract Attenuated NMDA-, H2O2, and TNF-α Induced Oxidative Response in Neurons, Astrocytes, and CECs
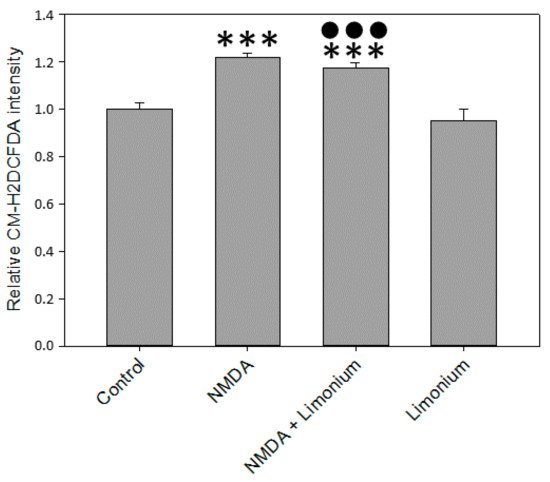
Cellular ROS production in human primary neurons, astrocytes, and CECs was quantified with CM-H2DCFDA, a fluorescein dye commonly used as an indicator for ROS in cells. In agreement with previously published reports
[8][18], our data indicated that in human primary neurons, 100 μM of NMDA increased CM-H2DCFDA fluorescence by 22% (
p ≤ 0.001) as compared with untreated control (see
Figure 2). At the same time, we observed a slight reduction of ROS (by 5%,
p ≤ 0.001) in the group pre-treated with plant extract compared to the untreated cells.
Figure 2. L. gmelinii extract inhibits ROS in human primary neurons exposed to NMDA. The level of ROS is represented by the relative intensity of CM-H2DCFDA normalized to the control group. ***—p ≤ 0.001 compared to the control; ●●●—p ≤ 0.001 compared to the group treated with NMDA.
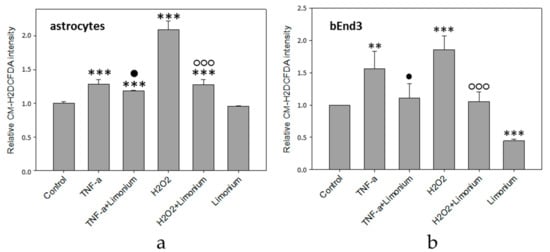
The results of quantitative fluorescence analysis of the level of ROS generation in astrocytes and bEnd3 cells exposed to TNF-α are presented in Figure 3. As a positive control, we exposed the cells to H2O2, which is a known source of ROS. According to the data obtained, the treatment of astrocytes with TNF-α and H2O2 resulted in a 28 and 100% increase in the production of ROS, respectively, as compared to control (p ≤ 0.001) (see Figure 3a). Pre-treatment of the cells with the extract from L. gmelinii prevented the accumulation of ROS in astrocytes. As compared to cells that were exposed to TNF-α or H2O2 alone, ROS indicator was reduced by 10 and 73%, respectively (p ≤ 0.01, p ≤ 0.001). At the same time, the extract itself did not influence the generation of ROS in astrocytes, as there was no significant changes in the fluorescence intensity of the dye in this group. Similarly, the treatment of bEnd3 cells with TNF-α and H2O2 resulted in a 56 and 86% increase, respectively, in the production of ROS (p ≤ 0.01, p ≤ 0.001), while the presence of L. gmelinii extract completely attenuated accumulation of ROS in these cells (p ≤ 0.05, p ≤ 0.001). Moreover, the Limonium extract itself was capable of significantly reducing the generation of ROS in bEnd3 cells (p ≤ 0.001), (see Figure 3b).
Figure 3. L. gmelinii extract reduces ROS in human primary astrocytes (a) and bEnd3 cells (b) treated with TNF-α and H2O2. The level of ROS is represented by the relative intensity of CM-H2DCFDA normalized to the control group. ***—p ≤ 0.001 compared to the control; **—p ≤ 0.01 compared to the control; ●—p ≤ 0.05 compared to the group treated with TNF-α; ○○○—p ≤ 0.001 compared to the group treated with H2O2.
It is worthy to note here that in bEnd3 cells treated with
L. gmelinii extract ROS was significantly lower than in control cells, whereas in astrocytes it remained the same as in control condition. This difference might be attributed to the existence of slightly different ROS-dependent downstream pathways for CECs (bEnd3 cells in particular) and astrocytes that we have reported previously
[19][20], albeit this is speculation.
4. Effect of L. gmelinii Extract on the Activity of NADPH Oxidase in Human Primary Astrocytes
To further access antioxidant properties of
L. gmelinii, we investigated the activity of NADPH oxidase in human primary astrocytes in the presence of TNF-α. NADPH-oxidase is a trans-membrane enzymatic complex comprising of two membrane-bound subunits (gp91-phox, p22-phox), three cytosolic subunits (p67-phox, p47-phox and p40-phox), and a low-molecular-weight G protein
[21]. Several researchers have demonstrated that NADPH-oxidase actively participates in the production of ROS in astrocytes
[19][22][23][24].
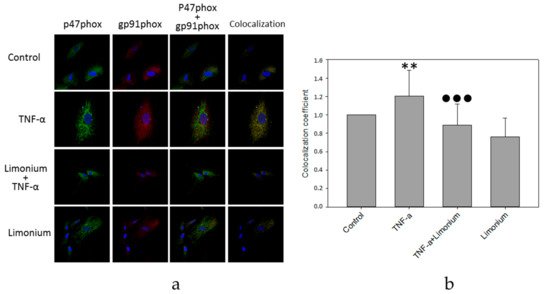
Since NADPH oxidase was activated by the assembly of the membrane-bound subunits with its cytosolic components, we tested the effects of
L. gmelinii extract on co-localization of gp91phox and p47phox subunits by analyzing confocal images of double immunofluorescent-labeled gp91-phox and p47-phox in rat primary astrocytes (see
Figure 4a,b).
Figure 4. L. gmelinii extract suppresses activation of NADPH-oxidase induced by TNF-α in human primary astrocytes. NADPH-oxidase activity is represented by the coefficient of gp91phox and p47phox subunits co-localization (normalized to the control group). Confocal images (a), quantitative analysis (b). **—p ≤ 0.01 compared to the control; ●●●—p ≤ 0.001 compared to the group treated with TNF-α.
As can be seen in Figure 4a, the number of yellow pixels in the combined images was minimal in the control group. In cells that were exposed to TNF-α, the number of yellow pixels increased markedly. In contrast, in the astrocytes, which were pre-incubated with polyphenol extract from L. gmelinii, and then with TNF-α, the level of yellow light glow on the combined images was much lower. Quantitative analysis showed that the level of co-localization of p47phox and gp91phox subunits of NADPH oxidase in astrocytes that were exposed to TNF-α increased by 20% (p ≤ 0.01), which indicates activation of this enzyme complex (see Figure 4b). For astrocytes that were pre-incubated with the extract, and then with TNF-α, the level of the co-localization of p47phox and gp91phox subunits was significantly lower (by 30%, p ≤ 0.001) compared to the cells that had only been exposed to TNF-α and remained at the control level suggesting either reversion or prevention of NADPH oxidase activation. The polyphenols extract itself had no significant effect on co-localization of the enzyme subunits.
5. Pretreatment with L. gmelinii Extract Suppressed Induced by TNF-α Pro-Inflammatory Responses in Astrocytes and CECs
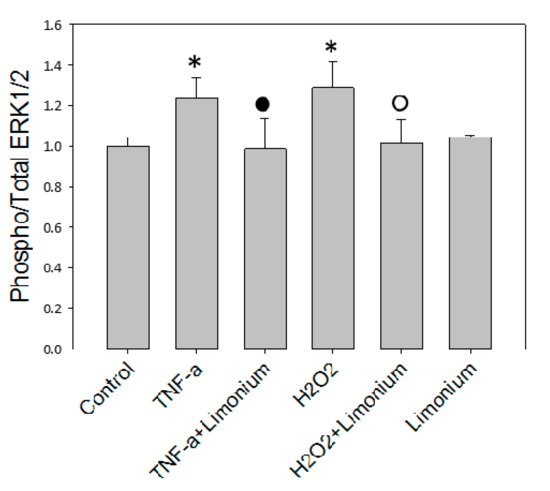
To assess the effect of the extract on the activation of ERK 1/2 under the influence of TNF-α, an analysis of the level of phosphorylation of this enzyme was performed in human primary astrocytes by using MAP kinase assay. The results of the ERK 1/2 protein kinase activity shown in Figure 5 demonstrated that the level of phosphorylation of ERK 1/2 was significantly higher (by 23.5%, p ≤ 0.05) in cells that were exposed to TNF-α compared to the control. If cells were pretreated with the extract phosphorylated enzyme content did not increase as much as in a TNF-α group, this suggested either reversion or prevention of ERK 1/2 activation. In this group, the amount of phospho-ERK 1/2 almost reached the control values. The extract itself did not affect the activity of the enzyme. The level of its phosphorylation in cells that were incubated only with the extract did not significantly differ from the control one.
Figure 5. L. gmelinii extract suppresses activation of ERK 1/2 kinase induced by TNF-α in astrocytes. *—p ≤ 0.05 compared to the control; •—p ≤ 0.05 compared to the group treated with TNF-α; ○—p ≤ 0.05 compared to the group treated with H2O2.
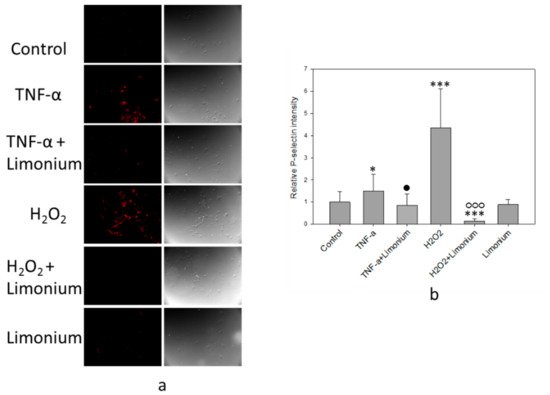
The results of immunofluorescence analysis of P-selectin mobilization are presented in Figure 6. Under the influence of TNF-α, the fluorescence intensity of the labeled P-selectin increased (see Figure 6a, colored red) as compared with the control, and in cells that had been pretreated with the extract, a decrease in the emission of the labeled P-selectin was observed. Quantitative analysis of the P-selectin content on the cell surface showed that, upon exposure to TNF-α, the P-selectin level increased by 50% (p ≤ 0.05) compared to the control. On the contrary, pre-incubation of the cells with plant extract prevented overexpression of the P-selectin on the surface of the cells (by 65%, p ≤ 0.05, compared to TNF-α only treated group). In cells that were incubated only with plant extract, the P-selectin content on the surface did not differ from the control level (see Figure 6b).
Figure 6. L. gmelinii extract suppresses TNF-α and H2O2-induced expression of P-selectin on the cell surface. Fluorescent images of P-selectin labeled bEnd3cells (a); relative intensity of P-selectin in the bEnd3 (b). ***—p ≤ 0.001 compared to the control; *—p ≤ 0.05 compared to the control; •—p ≤ 0.05 compared to the cells treated only with TNF-α; ○○○—compared to the cells treated only with H2O2.
We have previously reported that phosphorylation of ERK 1/2 in astrocytes and mobilization of P-selectin in CECs could be induced by ROS
[20][25]. As a positive control, in the present study, we have exposed the cells to H
2O
2 (see
Figure 5 and
Figure 6). In agreement with our earlier studies, exposure of the cells to H
2O
2 resulted in a 32% increase of Phospho/Total ERK 1/2 ratio (
p ≤ 0.05, compared to control) in human primary astrocytes and 4 fold increase of the P-selectin levels in CECs (
p ≤ 0.001, compared to control), while pretreatment with the plant extract either prevented or reversed phosphorylation of ERK 1/2 (
p ≤ 0.001, compared to H
2O
2) and overexpression of P-selectin on the surface of CECs (
p ≤ 0.001, compared to H
2O
2).
6. L. gmelinii Extract Improved Motor Activity in Rats with MCAO
As mentioned above, to study the neuroprotective effect of the extract from L. gmelinii on a model of laboratory animals, ischemic stroke was induced in male Wistar rats by occlusion of the middle cerebral artery (MCAO). The next day after the induction of the stroke, the animals started to receive an extract from the L. gmelinii at 200 mg/kg, intragastrically for 28 days.
In all the operated animals, in the first hours after waking up and on the first day of the postoperative period, development of neurological deficiency was observed, manifested by the development of lethargy and slowed movements, unilateral ptosis of the eye on the affected side, paresis or paralysis of the right anterior and/or hind paws, tremor, hemianesthesia of the affected side, suppression of appetite, dysfunction of the regulation of defecation and urination, and deterioration of sensorimotor activity (see Figure 7).
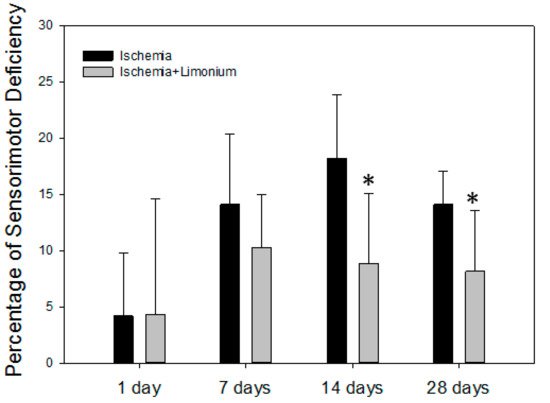
Figure 7. L. gmelinii extract improves motor coordination of rats with induced ischemic stroke after 14 and 28 days of treatment. The percentage of motor deficiency was calculated and normalized to the control trials (before surgery, represented as a 0 point). *—p ≤ 0.05 compared to the animals with ischemia only.
On the seventh day after the beginning of the experiment (MCAO), both groups of the animals showed an increase in sensorimotor disturbances: by 14.1% in the control group and by 10.3% in the treatment group. In control, a sensorimotor deficiency was increased on the 14th day (by 18.2%) and was still present on the 28th day (14.1%). In contrast, we did not observe negative dynamics on days 14 and 28 in animals treated with plant extract; moreover, motor activity was significantly improved in this group (by 6.8 and 4.5% compared to untreated control, p ≤ 0.05).