1000/1000
Hot
Most Recent

Medicinal mushrooms are increasingly being recognized as an important therapeutic modality in complementary oncology. Until now, more than 800 mushroom species have been known to possess significant pharmacological properties, of which antitumor and immunomodulatory properties have been the most researched. Besides a number of medicinal mushroom preparations being used as dietary supplements and nutraceuticals, several isolates from mushrooms have been used as official antitumor drugs in clinical settings for several decades. Various proteomic approaches allow for the identification of a large number of differentially regulated proteins serendipitously, thereby providing an important platform for a discovery of new potential therapeutic targets and approaches as well as biomarkers of malignant disease. This entry is focused on the current state of proteomic research into antitumor mechanisms of some of the most researched medicinal mushroom species, including Phellinus linteus, Ganoderma lucidum, Auricularia auricula, Agrocybe aegerita, Grifola frondosa, and Lentinus edodes, as whole body extracts or various isolates, as well as of complex extract mixtures.
Cancer ranks as the leading cause of death overall, while being the first or second leading cause of death before the age of 70 years in 112 of 183 countries [1]. It is known that cancer poses the highest clinical, social, and economic burden in terms of cause-specific disability-adjusted life years (DALYs) among all human diseases, followed by ischemic heart disease and stroke. The overall risk of developing cancer from age 0–74 is 20.2% (22.4% in men and 18.2% in women) [2]. Cancer incidence and mortality is rapidly growing worldwide, which reflects both population aging and growth as well as changes in prevalence and distribution of the main risk factors for cancer. In 2020 alone, 19.3 million new cases and 10 million cancer deaths were estimated. Overall, the five most commonly diagnosed cancers are female breast (11.7%), lung (11.4%), prostate (7.3%), nonmelanoma of skin (6.2%), and colon (6%) cancers. Lung cancer is the leading cause of cancer death (18% of total cancer deaths), followed by colorectal (9.4%), liver (8.3%), stomach (7.7%), and female breast (6.9%) cancers [1].
Cancer is a generic term that designates a large group of diseases that are characterized by sequential and/or simultaneous alteration of molecular pathways associated with cell proliferation, survival, differentiation, and death. Although cancer implies a heterogeneous group of diseases, which differ in the tissue of origin and by the cellular and molecular processes through which they originated, the basic features of tumors were formulated by Hanahan and Weinberg [3], where they defined six basic features common to all tumors, and subsequently expanded them with four more properties that allow tumor progression [4]. The basic six characteristics are the acquisition of the ability for autonomous and unrestricted growth (self-sufficiency in growth signals), avoidance of growth inhibition signals, evading apoptosis, unlimited replicative potential, formation of new blood vessels (sustained angiogenesis), and tissue invasion and metastasis. Additional features include genomic instability and tumor-stimulating inflammation, reprogramming of energy metabolism, and avoidance of the immune system.
While modern scientific research on medicinal mushrooms began during the 1960s in Japan, their traditional medicinal use has been known to exist for about 7000 years in China, India, Japan, and Korea [5]. Mushrooms can be defined as macro-fungi having fruiting bodies that are either hypogeous (underground) or epigeous (above the ground) [6]. Of about 7000 edible mushroom species, around 800 are known to possess pharmacological properties [7]. Medicinal mushrooms are known as a rich source of high- and low-molecular weight bioactive compounds (polysaccharides, polysaccharide-proteins/peptides, peptidoglycans, alkaloids, lectins, lipids, phenolics, polyketides, proteins, steroids, terpenoids, ribosomal, and non-ribosomal peptides etc.), which possess more than 130 therapeutic effects (cytotoxic, mitogenic, immunomodulatory, antiviral, antibacterial, hepatoprotective, hypocholesterolemic, hypoglycemic etc.) [8]. While high molecular weight compounds such as polysaccharides and polysaccharopeptides are primarily known for their immunostimulatory and immunomodulatory action, a large number of species-specific low molecular weight compounds are implicated in direct regulation of cancer signaling, such as nuclear factor-kappa B (NF-κB), mitogen-activated protein kinase pathway (MAPK), Akt, Wnt, Notch, and p53 pathways [6][8]. Due to a large number of pharmacologically active compounds present in certain medicinal mushrooms, they are regarded as potential multi-target therapeutics. This approach is especially important with complex diseases such as cancer, where pleiotropy of cancer pathways is one of the important factors in unsatisfactory effects of certain targeted therapies in the clinic, such as MMP inhibitors, as well as therapeutic resistance [9].
Medicinal mushrooms comprise a complex system of chemical components that have the potential to regulate multiple processes through multiple targets simultaneously. Proteomics is a large scale study of proteins, which is characterized by a hypothesis-free and comprehensive approach to studying novel mechanisms of potential therapeutics. Specifically, differential proteomics, also known as comparative or functional proteomics, studies the changes in proteome in different physiological or pathological states between two or more samples [10]. Cancer proteomics encompasses the identification and quantitative analysis of healthy tissue from neoplasia and can be used to identify markers for cancer diagnosis and treatment (biomarkers), monitoring disease progression, and identifying therapeutic targets. Despite its complexity, proteomics is necessary for accurate characterization of pharmacological action. One gene can potentially produce a large number of protein products, because of differential splicing as well as more than 200 posttranslational modifications that proteins can undergo, which affect their function, stability, and protein–protein and other interactions [11].
One of the sources of antitumor polysaccharides are mushroom spores. G. lucidum spore polysaccharides induce MAPK pathway and spleen tyrosine kinase Syk-dependent TNF-α and interleukin-6 secretion in murine peritoneal macrophages [12]. Ma et al. [13] demonstrated that Ganoderma lucidum spores (GL-SP) could stimulate splenic mononuclear cells (MNCs) proliferation and cytokine production. GL-SP was characterized by high-performance liquid chromatography (HPLC) and seven monosaccharides were identified. MNCs were obtained from inbred KM mice spleen. The proliferation of MNSc treated with 200, 400, or 800 μg/mL of GL-SP for 72 h showed a dose-dependent increase in proliferation. GL-SP also increased the production of IL-2 and TNF-α, although the effect on TNF-α production was more pronounced than on IL-2 production. In order to further investigate the differential protein expression between GL-SP treated (400 μg/mL) and untreated cells, 2-DE was conducted to separate the proteins, and 10 protein spots that exhibited > 2-fold increase or decrease in abundance were further identified by MALDI-TOF MS/MS analysis. Based on their biological functions, these 10 proteins were classified into three categories. Two proteins included in cell viability and proliferation included 14-3-3-tau (theta) protein and apoptosis-associated speck-like protein containing a CARD (ASC), which were both downregulated. Since 14-3-3 tau protein is involved in mitogenesis, cell cycle control (G1-S and G2-M cell cycle progression), and apoptosis, its downregulation may inhibit the apoptosis cascade and increase the number of viable mononuclear cells [14]. ASC protein is essential in intrinsic mitochondrial apoptosis pathway, so its downregulation protects MNCs from apoptosis [15]. Five proteins involved in cell activation and motility were found to be differentially downregulated as a response to GL-SP treatment. Upregulated T-cell-specific GTP-ase plays a role in the activation of lymphocytes induced by GL-SP [16]. Copine I protein, which is involved in apoptosis and TNF-α signaling pathway, was downregulated [17]. Phosphatidylinositol transfer protein α (PITP alpha) modulates cellular responses of lyphocytes to LPS and other mCD14 ligands, so its upregulation may contribute to the immunomodulating activity of GL-SP [18]. Rho, GDP dissociation inhibitor beta, has important roles in the maintenance of marginal zone B cells and retention of mature T cells in thymic medulla, so its upregulation is clearly indicative of its role in immunomodulating effects of GL-SP [19]. Upregulated myosin regulatory light chain 2-A mediates the effect of GL-SP on lymphocyte motility. Three proteins involved in cytoskeleton structure, maintaining cell shape and motility (beta actin, gamma actin, and tubulin alpha), were all downregulated, which could indicate cytoskeletal remodeling in lymphocyte activation [20].
Mushrooms produce a large number of biologically active proteins including lectins, ribosome inactivating proteins (RIPs), fungal immunomodulatory proteins (FIPs), and laccases [21]. Fungal immunomodulatory protein Ling Zhi-8 (LZ-8) is one of the most important bioactive substances of G. lucidum [22]. Lin et al. [23] studied the proteomic profile of LLC1 lung cancer cells after treatment with LZ-8 from G. lucidum . It was previously established that LZ-8 has antitumor roles in lung cancer [24], so this study was aimed at revealing the mechanisms of its antitumor action by differential proteomics. C57BL/6 mice were inoculated with 2 × 10 5 LLC1 cells. Control group was treated with PBS i.p., while the second group was treated with i.p. LZ-8 (7.5 mg/kg) on days 3, 7, 11, and 15. On day 17, tumor tissues were extracted and used for proteomic analysis. After 2-DE separation, protein identification by ESI-MS/MS revealed 21 differentially expressed proteins in comparison with control. Proteins with a value of p ≤ 0.05 and fold change ≥ 2 were deemed to be significantly differentially expressed. It was found that significantly downregulated proteins included various heat shock proteins (HSPs), T-complex protein 1, cytoskeleton-related proteins (tubulin, vimentin), protein disulfide-isomerase (PDIA3), and serum albumin [23]. Bioinformatic analysis (Ingenuity Pathway Analysis) revealed that a highly significant overlap of 15 canonical pathways was found and was connected with aldosterone signaling, protein ubiquitination pathways, and 14-3-3-mediated signaling. KEGG annotation revealed that 4 of the 21 proteins, GRP78 (Bip), HSP70, HSP90, and PDI-related proteins were included in protein processing/endoplasmic reticulum stress pathway. Heat shock proteins are a group of chaperone proteins whose expression is often increased in various cancer cells, such as lung cancer [25]. HSP90 stabilizes various oncoproteins, such as EGFR, HER2, ALK, and KRAS, while HSP70 inhibits apoptosis [26]. LZ-8 effectively reduces levels of various HSPs. The tested FIP also inhibited cancer cell viability, suppressed cell migration, and induced apoptosis by HSP downregulation, as was determined by Transwell and Western blot assays. It is known that HSP90 contributes to EGFR stabilization. Previous research demonstrated that LZ-8 effectively downregulates EGFR protein, which supports the finding that HSP downregulation may contribute to cellular apoptotic response [24].
Yue et al. [27] studied the effects of ganoderic acid D on the proteome of human cervical carcinoma HeLa cells. GAD was isolated and purified from G. lucidum and further purified by HPLC to obtain at least 99% purity. HeLa cells were incubated with 10 μM of GAD for 48 h and separated by 2-DE. Protein spots with 2-fold or more increased or decreased intensity with respect to control were subjected to further identification by MALDI-TOF MS/MS. Cytotoxic effects of GAD on HeLa cells in a range of concentrations from 1–50 μM for 24, 48, and 72 h was observed, proving to be dose- and time-dependent. Since 10 μM was the lowest concentration at which GAD induced both G2/M arrest and apoptosis, this concentration was chosen for protein analysis. Seven downregulated and 14 upregulated protein spots were identified. Proteins including eIF5A (eukaryotic translation initiation factor 5A-1) and spermidine synthase are important in cell survival and proliferation, and their observed downregulation after GAD treatment indicates their possible connection with cell growth inhibition [27]. Contrary to usual findings in both cervical and endometrial carcinoma, the expression of annexin A5 was increased as a result of GAD treatment [28]. Annexins are important in several biological processes, including membrane trafficking, proliferation, differentiation, and apoptosis, and are important positive or negative prognostic biomarkers, depending on the cancer type [29][30]. 26 S proteasome subunit p40.5, which is an important subunit of proteasomes, was increased after GAD treatment, which might contribute to possible protein degradation of HeLa cells. Ephrin receptor EphA7, thioredoxin-dependent peroxide reductase mitochondrial precursor, activator of heat shock 90-kDa protein ATPase homolog 1, ubiquinol-cytochrome c reductase core I protein, protein-disulfide isomerase, aminopeptidase B, and mitofilin are enzymes or regulators of enzymes that play important roles in cell metabolism, and whose change in protein expression indicated changes in metabolism of HeLa cells as a result of treatment. A member of the peroxiredoxin family of antioxidant enzymes PRDX3, which is an important component of antioxidant defense system and mitochondrial homeostasis, was upregulated after GAD treatment of HeLa cells. This indicates its possible role in HeLa cells growth inhibition, since PRDX3 overexpression has been correlated to decreased cell growth [31]. GAD-induced apoptosis may also be induced by several cytoskeleton-related proteins that were found to be downregulated, including microtubule-associated protein RP/EB family member 1, cytokeratin 19, cytokeratin 1, and calumenin. Namely, these proteins participate in cell cycle control and apoptosis, while cytokeratin 19 expression is known to be elevated in cervical carcinoma [27][32]. The authors found that the 14-3-3 family of proteins may have an important role in the cytotoxicity mechanism of GAD, since they were upregulated. Moreover, the identification of potential protein targets for GAD by INVDOCK program revealed that six members of 14-3-3 protein family were predicted to be able to bind directly to GAD.
Furthermore, Yue et al. [33] subsequently analyzed the proteomic profile of HeLa cells treated with five purified ganoderic acids: ganoderic acid F (GAF), ganoderic acid K (GAK), ganoderic acid B (GAB), ganoderic acid D (GAD), and ganoderic acid AM1 (GAAM1) ( Figure 2 ). The purity of the ganoderic acids was more than 98%. Based on the IC50 value obtained through cytotoxicity assay, which was about 15 μM for all ganoderic acids (GA), HeLa cells were incubated with the aforementioned concentration of either GA for 48 h. Protein spots with 2-fold or more increased intensity and statistically significant in each ganoderic acid-treated group were chosen for identification by MALDI-TOF MS/MS. Among the protein spots that were differentially expressed in each ganoderic acid-treated group, 12 protein spots were found to show similar change tendency in all ganoderic acids-treated groups compared with control. These 12 differentially expressed protein spots were identified by MS/MS. These 12 possible target-related proteins of ganoderic acids could be classified into four categories according to their biological function: cell proliferation or cell death, carcinogenesis, oxidative stress, and calcium signaling and endoplasmic reticulum (ER) stress. Tue same as was discovered in their previous research, one of the downregulated proteins related to cell proliferation and/or cell death was eIF5A, which functions as an elongation factor while 14-3-3 beta/alpha proteins are upregulated [34]. Ubiquilin 2, which modulates proteasome-mediated protein degradation, thus increasing their half-life, was downregulated. PP2A subunit A RP65-alpha isoform, a subunit of PP2A (protein phosphatase 2), which is essential for cell survival, cell cycle regulation, and DNA damage response, was downregulated. Proteins from the second group are the carcinogenesis-related proteins, which are differentially expressed in tumor in comparison with normal cells or tissues. Interleukin-17E, which has a role in T-cell-mediated angiogenesis, and heterogeneous nuclear ribonucleoprotein K (HNRPK), with roles in mRNA splicing and processing, were downregulated as a result of GA treatment [35][36]. Proteins that have important roles in oxidative stress, namely peroxiredoxin 2 (PRDX2) and DJ-1 protein chain A, were both downregulated. Since they both have functions in reducing oxidative stress, it can be hypothesized that they could be involved in ROS-mediated tumor cell death [37][38]. Cancer cells have an increased ROS level compared to normal cells due to high metabolic rate and mitochondrial dysfunction, which render increased susceptibility to oxidative stress [39]. Nucleobindin-1 is a protein involved in ER stress by regulating a function of activating transcription factor 6, an ER membrane-anchored transcription factor [40]. Reticulocalbin 1 is involved in the regulation of calcium-dependent activities in the ER lumen [41]. The authors concluded that eIF5A, 14-3-3 protein, and peroxiredoxin might be the most important target-related proteins of ganoderic acids [33].
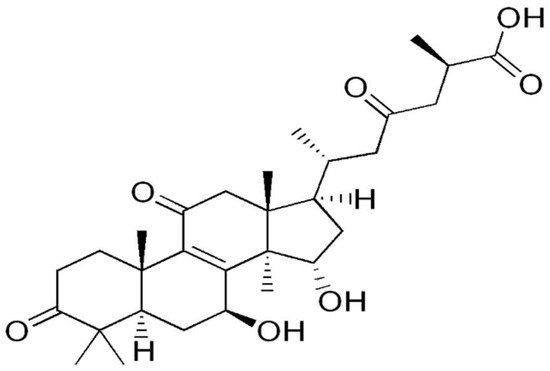
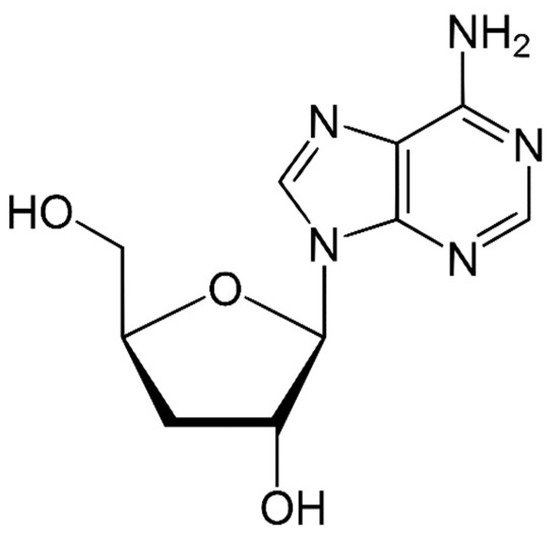
Species of the genus Cordyceps are entomopathogenic Ascomycete mushrooms, which are widely used in traditional medicine. Various Cordyceps species have been identified for their pharmacological properties, including C. sinensis , C. militaris , and C. pruinosa . Some of the studied active ingredients include cordycepin, cordycepic acid, sterols (ergosterol), nucleosides, and polysaccharides [59]. Cordycepin, or 3′-deoxyadenosine, has significant antitumor activities, which include inhibition of cell proliferation, migration, and induction of apoptosis [60][61] ( Figure 4 ). Its effects are dose-dependent; at low doses, it interferes with mRNA production and assembly of proteins, thus inhibiting uncontrolled cell growth and division. At higher doses, it inhibits cell adhesion and blocks protein synthesis through its effects on Akt and 4EBP phosphorylation [62].
Jeong et al. [59] studied the effects of Cordyceps militaris fresh fruit bodies or mycelia on cisplatin-resistant A549/CR lung cancer cells. Cisplatin is usually the first-line chemotherapeutic for patients with advanced NSCLC (non-small cell lung carcinoma). Besides its high toxicity, one of the major clinical problems is cisplatin resistance. In this research, cordycepin was first quantified in the sample by HPLC. Qualitative components were further detected by LC-MS. Cell-viability assay showed a dose-dependent inhibition of A549/CR cell viability, with a IC50 = 0.57 ± 0.12 mg/mL. Afatinib, a tyrosine kinase inhibitor, which was used as a positive control, had IC50 = 2.3 ± 0.23 μg /mL. Flow cytometry revealed that time-dependent percentages of the live cells were decreased from 78.32 to 17.63, which was shown to be the consequence of elevated initiator caspases-8 and -9, and executioner caspase-3, and was more prominent in cordycepin (CME) vs. afatinib-treated cells. Cell-cycle analysis demonstrated that CME increased the percentage of sub-G1 A549/CR-treated cells to 33.2 ± 1.2%, compared with Afatinib (17.6 ± 0.6%) and vehicle-treated control group (13.3 ± 1.6%) at 48 h. This demonstrated that CME increased accumulation in S phase in A549/CR cells, which was followed by cancer cell apoptosis. Proteomic profiling was done using a protein chip-based antibody array after treatment of A549/CR cells with 1.5 mg/mL CME. Proteins having a normal median ratio in the range of 1.0 were considered as unchanged expression. Among the cell-cycle proteins analyzed (42 of them), the only protein that exhibited a change in expression levels was H-Ras, which was significantly downregulated. It is well-known that H-Ras is a crucial protein that promotes cell proliferation by regulating cell cycle progression in most cancers [63]. In conjunction with previous results, it is clear that CME controls cell cycle progression by inhibiting Ras downstream signaling, such as Raf/MEK/ERK and PI3K-Akt, which results in suppressing the proliferation of A549/CR cells. The authors suggested that CME showed the possibility of overcoming the cisplatin resistance in NSCLC [59].
Cai et al. [64] investigated the effects of Cordyceps sinensis extracts on 4T1 breast cancer cells in vitro and in vivo. It was determined that one of the major components of water extract of Cordyceps sinensis (WECS) is polysaccharide, which constitutes 19.83% of extract ( w / w ). Nucleosides were also among the main components of WECS, and their content was analyzed by HPLC. Cytotoxicity assay showed that WECS had a significant effect on reducing 4T1 cell viability, being approximately 50% at 0.40 mg/mL. Since metastases present one of the main problems in clinical cancer management, the anti-metastatic effect of WECS was studied specifically. In the metastasis model, 1 × 10 6 4T1 cells were injected into the tail vein of Balb/c mice. From the day of tumor inoculation, the mice were intraperitoneally injected with 50 mg/kg/day WECS or vehicle for 15 days. Kaplan– Meier analysis, which was followed up for 33 days post 4T1 injection, showed a significant improvement in mice survival treated with 50 mg/kg/day of WECS. The number of metastatic lung nodules was also significantly reduced in the same group (about 10 per mice), both in their size and number, compared with untreated control (about 70 nodules per mouse). Further evidence of anti-metastatic effect is the 50% reduction in MMP-9 concentration after administration of WECS. Protein analysis of the lung tissue homogenates was done by protein array, which compared the expression of 111 cytokines in treated and untreated 4T1 tumor-bearing mice. It was shown that 6 cytokines were upregulated more than 2-fold in 4T1 tumor-bearing mice compared to normal mice, namely OPN (osteopontin), CCL12 (chemokine (C-C motif) ligand 12), IL-33, CCL17, CCL6, and MMP-9. Of these OPN, IL-33, CCL17, and MMP-9 were significantly reduced in the lung of 4T1 tumor-bearing mice treated with WECS. It is known that various cytokines as well as growth and inflammatory factors have an important role in migration and colonization of metastatic tumor cells [65]. It is known that OPN can promote lung metastasis [66]. IL-33 is also known to promote breast cancer metastasis through increasing immunosuppressive cells [67]. CCL17 is important for homing of CCR4 positive regulatory T cells in the lungs [68]. The authors speculated that WECS can reduce DNA damage and DNA damage response (DDR), which is strongly correlated with immune response and promotes inflammation in late tumor stages through cytokine recruitment, which is in line with previous research on Cordyceps sinensis [69].
Wang et al. [70] investigated anticancer mechanisms of Cordyceps cicadae against hepatocellular carcinoma in vitro using a proteomic approach. A lyophilized hot water extract of wild-type C. cicadae was used to treat MGCC97H cells in various concentrations (0–1000 μg/mL), and dose-dependent inhibition was observed. Cell cycle analysis revealed that treatment with C. cicadae (at 100, 250 and 500 μg/mL) induced a G2/M cell accumulation and decreased the cell percentages in G0/G1 phase. The highest dose (1000 μg/mL) induced a G2/M arrest in 64.75% of the cells. After incubation of MHCC97H cells with 500 μg/mL for 48 h, cells were subjected to proteomic analysis. 2-DE revealed 28 proteins with significant ( p < 0.05) changes of >1.5 fold in volume intensity, which were selected and identified by MALDI-TOF-MS/MS. The major biological functions of these proteins are cell growth and cell cycle regulation, anti-cancer effects, and other functions (cell redox regulation, protein folding, mRNA splicing). 14-3-3 gamma is one of the proteins that regulates the cell cycle, and its downregulation is contrary to its usual overexpression in HCC [71]. 14-3-3 downregulation could also account for G2/M phase arrest. BUB3 (mitotic checkpoint protein BUB3 isoform A), DCTN2 (Dynactin subunit 2), and MAPRE1 (microtubule-associated protein RP/EB family member 1) are involved in spindle checkpoint and mitosis regulation, and their deregulation may also contribute to G2/M phase arrest [70]. GLRX (thioredoxin-like protein) and CLIC1 (chloride intracellular channel protein 1) also regulate cell growth so their dysregulation could account for G2/M phase arrest. Among the proteins with anti-cancer effects, the upregulation of HSPB1 (heat shock protein beta-1) could indicate some resistance of the cancer cells and the absence of apoptosis in the cells treated with C.cicadae . Upregulation of ENO1 (alpha-enolase isoform 1) can result in the promotion of hepatocellular carcinoma through oxidative stress and protein misfolding [72]. ERP29 (Protein-Disulfide Isomerase Related Chaperone Erp29) is active in the endoplasmic reticulum, so its reduced expression can be attributed to reduced ER stress after treatment with C. cicadae. ER stress and unfolded protein response are involved in HCC development, aggressiveness, and response to treatment [73]. Downregulation of STRAP (WD-40 repeat protein), which is involved in pre-mRNA splicing, has a positive prognostic significance, because its overexpression was reported in several cancers [74]. Another protein that was downregulated as a result of treatment with C. cicadae is peroxiredoxin 1 (PRDX1). The elevated expression of PRDX1 was found in various cancers and its downregulation might facilitate a failure of the endogenous antioxidant systems, which protect cancer cells from ROS [75].
Mushrooms of genus Pleurotus, or oyster mushrooms, comprise about 40 species of lignocellulosic mushrooms. Recently, various molecules with pharmacological properties have been identified, including those with anti-neoplastic and immunomodulating effects (including α- and β-glucans, lentanin, resveratrol, POMP-2 ( Pleurotus ostreatus mycelium polysaccharide 2), POPS-1 (polysaccharide obtained from the fruiting body of Pleurotus ostreatus ), concavalin A, cibaron blue affinity protein), and antioxidant activity (pleuran, ergosta-7). The most studied species include P.ostreatus , P. eryngii , P. nebrodensis , P. citronopileatus , and P. sajor-caju [76].
Finimundy et al. investigated the antitumor, specifically cell-death-inducing properties of P. sajor-caju on colorectal cancer model [77]. Pleurotus sajor-caju fruiting bodies extracts were obtained using various solvents [n-hexane, chloroform, ethyl acetate, ethanol and ethanol/water (1:1, v / v )]. HCT-116 colon adenocarcinoma cell line can be classified as a consensus molecular subtype 4 (CSM4, mesenchymal). According to this classification, which is of importance in preclinical research, CSM4 tumors are those that are diagnosed at more advanced stages (III and IV) [78]. In this research, HCT-116 wt , -Bax , -p21 , and -p53 were used in order to correlate the observed anti-proliferation activity to activation of pro-apoptotic and/or cell arrest regulation pathways. Also, MRC-5 healthy lung fibroblast cell line was used in order to verify the cell selectivity of the treatment. n-hexane extract of Pleurotus sajor-caju (PSC-hex) was chemically characterized by GC-MS. The viability assay (MTT) confirmed that the most significant results were obtained with n-hexane extract (PSC-hex) on HCT-116 wt cells (IC50 = 0.05 mg/mL), followed by the PSC acetone extract on the same wild type cell line. Meanwhile, n-hexane extract showed practically no anti-proliferative activity on the MRC-5 cell line. The authors hypothesized that this selectivity might be explained by inhibition of the mitochondrial complex I, which is a target of lipophilic compounds that are extracted by n-hexane. On the other hand, no anti-proliferative activity was observed in Bax (HCT-116 -Bax ), p21 (HCT-116 -p21 ) or p53 (HCT-116 -p53 ) deficient cell lines, indicating that PSC-hex promotes its cytotoxicity by inhibiting tumor-associated signaling pathways. Flow cytometry analysis showed that, after treatment with 0.05 mg/mL of PSC-hex, the number of HCT-116 wt cells in early apoptosis increased from 0.45% to 65.6%, while the number of viable cells decreased from 92.2% to 25.9%. Furthermore, cell cycle analysis has shown that PSC-hex induces G2/M cell cycle arrest, and a significant accumulation of cells in the sub-G1 fraction, which indicates apoptosis induction. Therefore, it was assumed that PSC-hex exerts the observed cytotoxicity through a pro-apoptotic pathway. PSC-hex caused a loss of mitochondrial membrane potential (ΔΨm), which is followed by cytochrome c release and the activation of caspase-9, which indicated an internal apoptosis pathway activation. Many chemotherapeutics are selectively toxic to tumor cells because they increase oxidative stress i.e., ROS levels in tumor cells (which are already characterized by higher ROS levels than normal cells) above the levels that antioxidant cell mechanisms can resolve [79]. Flow cytometry using DCF-DA stain showed that treatment with PSC-hex causes 3-fold (0.025 mg/mL) and 2-fold (0.05 mg/mL) increase in H 2O 2 and O 2•− levels, respectively. Proteomic analysis by Proteome Profiler Array (43 proteins studied) revealed that there was an increased expression of several apoptosis-related proteins, including Fas, HSP60, HSP70, Xiap, HTRA, Survivin, Smac, caspase-3, cytochrome-c, p52, Bax, Bad, Bid, and Bim. Docking simulation demonstrated that one of the main identified compounds in the PSC-hex extract, ergosta-5,7,22-trien-3β, fits into the Bcl-2 hydrophobic cleft, indicating a possibility of an alternative pathway for inducing apoptosis by direct compound interaction.
Ma et al. [80] analyzed the proteome alterations in RAW 264.7 macrophages as a result of treatment with PEP 1b, a novel immunoregulatory protein isolated from Pleurotus eryngii . A previous study obtained and identified this protein (PEP) with a molecular weight of 21.9 kDa and informed that it could boost cellular immune response through cytokine and NO (nitric oxide) secretion. This study identified Toll-like receptor 4 (TLR4) as the receptor for this protein [81]. RAW 264.7, a murine macrophage cell line, was pretreated with medium and then treated with various concentrations of PEP 1b (0, 50, 100, and 200 μg/mL). The overall assessment of proteomic regulation in the PEP 1 b treatment group vs. control group was done using iTRAQ-based protein quantification approach. Three comparison groups were established: PEP 50/CT, PEP 100/CT, and PEP 200/CT, which represented the differences in expression of reliable proteins between 50, 100, and 200 μg/mL PEP 1b treatment groups and control group, respectively. A total of 2277 reliable proteins from RAW 264.7 macrophage cells were identified. Differential proteins were those with an average fold change (FC) of more than 1.2 or less than 0.83 in treated groups compared to control. The numbers of detected differential proteins (DEP) increased with PEP concentration; PEP 50/CT group: 116 proteins (57 upregulated and 59 downregulated), PEP 100/CT group: 165 proteins (89 upregulated and 76 downregulated); and PEP 200/CT group 292 proteins (191 upregulated and 101 downregulated). The expression level of some proteins was stepwise increased with the concentration increase of the PEP 1b treatment, which points to a dose-response relationship: macrophage migration inhibitory factor (Mif), interferon-induced transmembrane protein 3 (Ifitm3), cyclooxygenase 2 (Cox2), Ras-related protein 1b (Rap1b), and sequestosome 1 (Sqstm1). The further analysis of the protein data was focused on PEP 200/CT group. On the basis of GO biological process analysis, differential proteins were mainly attributed to small-molecule metabolic process (18%), immune system process (15%), cellular catabolic process (14%), oxidation reduction process (13%), and inflammatory response (5%), which were associated with the immune-boosting activity of the macrophage. KEGG analysis via heat maps demonstrated that there were four pathways that were upregulated with the increase of the concentration of PEP 1b treatment: immune system, transport and catabolism, carbohydrate metabolism, and signal transduction. KEGG analysis showed that PEP 1b could upregulate immunoregulatory pathways, such as NF-κB, VEGF, and TNF pathways, but also the following pathways: hedgehog signaling pathway, sphingolipid signaling pathway, Rap1 signaling pathway, Wnt signaling pathway, phospholipase D signaling pathway, PI3K−Akt signaling pathway, and Ras signaling pathway. The proteins associated with the NF-κB signaling that was upregulated was Sqstm1, which functions as an adaptor protein in concert with TNF receptor-associated factor 6 to regulate the activation of NF-κB in response to upstream signals [82]. Upregulated Cox2 can also positively regulate the NF-κB nuclear transfer process to mediate the immune response of macrophages [83]. PEP 1b also upregulated the expression of Mif (macrophage migration inhibitory factor), Rap1b (Ras-related protein Rap-1b), transmembrane glycoprotein NMB (Gpnmb), superoxide dismutase (Cu−Zn) (Sod1), C5a anaphylatoxin chemotactic receptor 1 (C5ar1), and peroxiredoxin 2 (Prdx2), which modulate the MAPK pathway. Mif is important in cell-mediated immunoregulation and inflammation as well macrophage function through suppression of anti-inflammatory effects of glucocorticoids [84]. Rap is a protein of the Ras family that affects T cells through integrin and modulates cell adhesion [85]. Peroxiredoxin 2 is a member of antioxidant enzymes that reduces hydrogen peroxide and alkyl hydroperoxides, and activates MAPK signal pathway [86]. The proteomic analysis also revealed that PEP 1b modulated the nitric oxide biosynthetic process through upregulation of Cox2, heat shock protein (Hsp90aa1), Pyk, and Itgb2 (integrin-beta 2). Pyk2 (protein tyrosine kinase 2 beta) is involved in multiple immune signaling pathways (JNK/MAPK, Akt/MAPK, and JNK/SAPK) [87]. This research thus demonstrated that PSP 1b protein can influence critical proteins important in immunoregulatory activities in macrophages.