Plant polyphenols are the main category of natural active substances, and are distributed widely in vegetables, fruits, and plant-based processed foods. Polyphenols have a beneficial performance in preventing diseases and maintaining body health.
1. Classification, Source and Function of Polyphenols
More than 8000 phenolic substances are commonly distributed in fruits, vegetables, tea, coffee, cocoa, beans, and grains (
Table 1)
[1]. Polyphenols have complex structures and can be divided into phenolic acids, lignans, stilbene, tannins, and flavonoids (e.g., isoflavones and anthocyanins). Polyphenols derived from various sources have many beneficial and specific therapeutic properties (
Table 1). Phenolic acid has an extensive physiological activity, including anti-oxidation, scavenging free radicals, anti-ultraviolet radiation, and antibacterial and antiviral effects. Stilbene resveratrol has a preventive effect on atherosclerosis and cancer
[2]. Stilbene and flavonoids can be used to prevent and treat cardiovascular and cerebrovascular diseases
[3][4][5]. As the most common phytoestrogens, lignans are famous for its high anti-oxidant activity and inhibiting lipid peroxidation
[6][7]. Lignans can also bind to estrogen receptors and interfere with cancer-promoting effects; therefore, it has a preventive effect on breast and colon cancer. As a kind of polyphenols, tannin can exert various activities, such as anti-oxidative, anti-microbial, anti-cancer, anti-hypertensive, and anti-inflammatory effects
[8][9]. However, complexes can be formed by polyphenols with starch, protein, and enzymes; therefore, they are considered as anti-nutrients. Due to their carcinogenic and anti-nutritional effects, it is harmful for human and animal to have too many tannins
[10].
Table 1. Classification, sources, and functions of polyphenols.
| Polyphenols |
Subclass |
Sources |
Function |
Ref. |
| Phenolic acids |
|
Coffee, berries, kiwi, apple, cherry |
Anti-inflammatory, anti-oxidant, antibacterial, antiviral, antiparasitic |
[11][12][13] |
| Stilbenes |
|
Grapes, wine |
Anti-inflammatory, anti-oxidant, heart protection, anti-cancer, anti-obesity |
[14][15][16] |
| Lignans |
|
Linseed, sesame, wheat |
Anti-tumor, scavenging free radicals, anti-oxidant |
[17][18][19] |
| Flavonoids |
|
|
|
|
| |
Isoflavones |
Soy, miso |
Estrogenic activity, anti-inflammatory, anti-obesity, anti-diabetic, anti-oxidant, cholesterol lowering |
[20][21][22] |
| |
Flavones |
Parsley, celery, capsicum pepper |
Anti-inflammatory, anti-oxidant, regulating glucose and lipid metabolism, anti-virus, anti-bacterial, anti-parasitic |
[23][24][25] |
| |
Flavanones |
Grapefruit, lemon, oranges |
Anti-inflammatory, anti-oxidant, regulating glucose and lipid metabolism, preventing liver steatosis, anti-bacterial, anti-viral, anti-parasitic, anti-fungal |
[26][27] |
| |
Flavonols |
Berries, onion, broccoli, leek |
Anti-inflammatory, anti-oxidant, anti-virus, anti-bacterial |
[28][29][30] |
| |
Flavanols |
Grapes, cocoa, wine, apricots, green tea, beans |
Anti-inflammatory, anti-oxidant, antibacterial, antiviral, antiparasitic, anticancer |
[31][32][33] |
| |
Anthocyanins |
Berries, black grapes, aubergine, red wine, rhubarb |
Anti-inflammatory, anti-bacterial, anti-oxidant, anti-diabetic, anti-cancer, nerve protection, anti-allergic |
[34][35][36] |
| Tannins |
Condensed tannins |
Cocoa, chocolate, apples, grapes |
Anti-oxidant, eliminating free radicals, enhancing immunity, preventing cardiovascular and cerebrovascular diseases, improving hypoxia |
[37][38][39] |
| |
Hydrolyzable tannins |
Mango, pomegranate |
Anti-oxidant, anticancer, phytoestrogens activity |
[40][41] |
Due to their extensive biological activities, plant polyphenols have become a study hotspot in the field of human nutrition and health. Similarly, polyphenols also have various positive effects on livestock and poultry. Plant polyphenol extracts and polyphenol monomer compounds can effectively improve animal intestinal microenvironment with various functional activities, such as immune regulation, bacteriostasis, anti-oxidation, and microbiota regulation
[42][43].
2. Foodomics Applied in the Study of Polyphenols
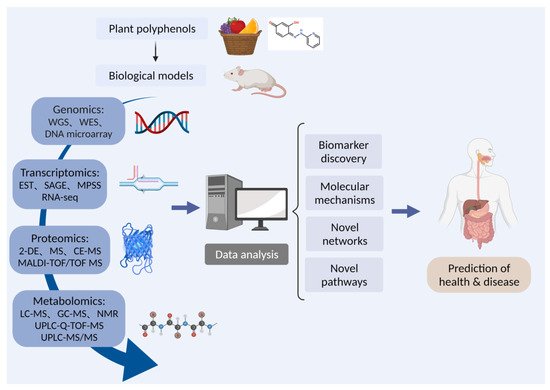
As shown in Figure 1, foodomics is a collection of genomics, transcriptomics, proteomics, and metabolomics, and can be used to study polyphenols from multiple angles. The data obtained from various omics will be integrated to explore the molecular mechanism and novel pathways of plant polyphenols to predict and treat diseases of human and animals.
Figure 1. The strategy of foodomics to study the bioactivities of polyphenols. 2-DE: two-dimensional gel electrophoresis; CE-MS: capillary electrophoresis mass spectrometry; EST: expression sequence tags technology; GC: gas chromatography; LC: liquid chromatograph; MPSS: massively parallel signature sequencing; MALDI-TOF/TOF: matrix-assisted laser desorption ionization time-of-flight/time-of-flight; MS: mass spectrometry; NMR: nuclear magnetic resonance; RNA-seq: RNA sequencing; SAGE: serial analysis of gene expression; UPLC-Q-TOF: ultra-performance liquid chromatography to quadrupole time-of-flight; WES: whole exome sequencing; WGS: whole genome sequencing.
3. Microbiomics Involved in the Bioactivity of Polyphenols
3.1. Regulation of Polyphenols on Gut Microbiota
Microbiome refers to all microorganisms and genetic information in a specific environment and has beneficial effects in nutrition, metabolism, and immunity
[44]. Gut microbiota mainly consists of
Actinobacteria,
Bacteroidetes,
Firmicutes,
Fusobacteria,
Proteobacteria, and
Verrucomicrobia. Among them,
Firmicutes and
Bacteroides are the dominant microbiota
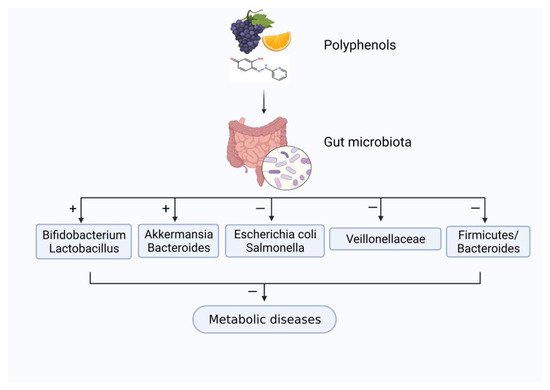
[45]. Like prebiotics, the polyphenols in diet have received widespread attention for their functional regulatory effects on gut microorganisms, as shown in
Figure 2. Polyphenols can inhibit harmful bacteria proliferation (e.g.,
Escherichia coli and
Salmonella), while promoting the growth of probiotics (e.g.,
Bifidobacterium and
Lactobacillus).
Figure 2. The influence of the interaction between plant polyphenols and gut microbiota on metabolic diseases. “+” means “enhance”; “−” means “weaken”.
Intestinal disorders can be inhibited by polyphenols via richening the abundance of beneficial bacteria and microbial diversity. Resveratrol has a promoting effect on
Lactobacillus and
Bifidobacterium, which can exert anti-inflammatory effects through reducing pro-inflammatory cytokines and increasing anti-inflammatory cytokines
[46][47]. Alpha diversity of gut microbiota was changed, and relative abundances of
Bifidobacterium,
Feacalibacterium,
Eubacterium, and
Coprococcus were increased by the intake of polyphenols from green tea
[48]. The abundance of
Bifidobacteria and
Lactobacillus in the gut were increased by the intake of blueberries
[49]. In addition, it has been confirmed that inflammatory bowel disease (IBD) is influenced by multiple factors, including the host, microorganisms, and the environment, and the occurrence of IBD is related to gut microbes
[50]. In our lab, we found that polyphenol taxifolin changed the composition of colonic microbial community by 16S rDNA sequencing. The change in
Bacteroides,
Clostridium saccharogumia,
Clostridium ramosum,
Sphingobacterium multivorum, and
Bacteroidetes/
Firmicutes ratio caused by dextran sulfate sodium was restored by taxifolin to relieve mice colitis
[51]. In conclusion, plant polyphenols can promote beneficial bacteria in the process of regulating intestinal microbes. Once the polyphenols enter the intestinal tract, they will activate the gut microbiota and regulate gut microecology. Conversely, polyphenols can also be used by gut microbiota to produce bioactive molecules (e.g., phenolic acids), which may be the key biologically active effector
[52][53], subsequently promoting the health of human and animals.
3.2. Combination of Microbiome and Metabolomics in Polyphenol Study
Currently, microbiome technologies mainly contain microbial metagenomics, metametabolomics, macrotranscriptomics, and macroproteomics, allowing us to analyze the microbiome at different levels (e.g., DNA, RNA, protein, and metabolites). At present, numbers of studies on polyphenols mainly focus on metagenomics and metametabolomics. In contrast, their combination to explore the metabolic process of polyphenols is still lacking. The combination of metabolomics and microbiome is a novel approach to explore the specific mechanism of polyphenols. Gut microbiota plays a critical role in health and nutritional status of human and animals
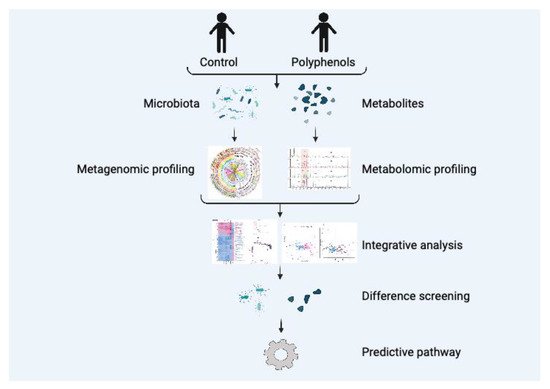
[54]. In general, the method of the combination of microbiome and metabolomics is shown in
Figure 3. By sequencing the metagenomics of gut microbiota, the corresponding microorganisms can be identified and metabolites can be analyzed using metabolomics technology to discover a novel pathway. In a previous study, primary bile acids were modified into secondary bile acids by clostridium species using 16S amplicon sequencing and metabolomics
[55]. It has been verified that polyphenols have various biological activities with a positive influences on gut microbes
[56][57][58]. Therefore, the combination of metabolomics and microbiome in the exploration of polyphenols is a trend in the future.
Figure 3. Combining microbiome and metabolomics to investigate the bioactivity of plant polyphenols.
The combination of microbiome and metabolomics has been used to study the effects of plant polyphenols on cardiovascular diseases. Through the metabolomics and genomics analysis of microorganisms in serum, urine, and feces, the risk of cardiovascular disease can be reduced by pomegranate polyphenols
[59]. The influence of green tea polyphenols on gut microbiota and micronutrient metabolism was analyzed using metagenomics and metabolomics, and the metabolites of tricarboxylic acid and urea cycle were analyzed using metabolomics and 16S rRNA sequencing, showing that energy conversion was enhanced by green tea polyphenols via promoting the metabolism of gut microbiota in rat
[60]. Moreover, the diversity and overall structure of gut microbiota were changed by polyphenols using 16S rRNA sequencing, indicating that polyphenols have an anti-cancer effect. Therefore, it has been speculated that polyphenols can regulate tumor growth by controlling certain bacteria and subsequently changing the cellular components and metabolites
[61]. In conclusion, the positive effects of polyphenols on human gut health can be clarified through a microbiome approach. The underlying mechanisms of polyphenols on gut microbiota and metabolites using microbiomics, metabolomics, and multiple omics need to be further explored.