Gold and Silver nanoparticles (AuNPs and AgNPs) are perfect platforms for developing sensing colorimetric devices thanks to their high surface to volume ratio and distinctive optical properties, particularly sensitive to changes in the surrounding environment. These characteristics ensure high sensitivity in colorimetric devices. Au and Ag nanoparticles can be capped with suitable molecules that can act as specific analyte receptors, so highly selective sensors can be obtained.
1. Introduction
Colorimetric methods draw attention due to their simplicity and low cost. Detection with these sensors is generally obtained merely by viewing with the naked eye the color changes, so no sophisticated or expensive additional devices are needed. Moreover, they can be used in field analysis and point-of-care testing
[1]. Rapid and cost-effective colorimetric devices have demonstrated their good performance in biosensing, environmental monitoring and medical diagnosis. The development of these sensors has rapidly grown in recent years; this trend shows the deep interest of the scientific community in this field. The challenge in developing new colorimetric sensors with a simple naked-eye detection of the color changes induced by the analyte. For this purpose, several chromogenic substrates and color labels have been explored, particularly those with high selectivity, sensitivity, low cost and suitable for practical applications. Among them, dye-based and nanoparticles-based sensors have been widely studied
[2][3].
Traditionally, dye-based colorimetric devices have attracted great attention thanks to their low cost, high stability, and usage for several applications, such as chemical and biochemical sensing, medical diagnosis and environmental analysis
[1]. The functional principle of these colorimetric sensors depends on the color changes induced or mediated by an analyte after its reaction with the dye. Unfortunately, these methods are not very sensitive since the generally low extinction coefficients of the dyes and the scarce accuracy in perceiving small color variations by the naked eye. Moreover, it is often difficult to determine individual components of a mixture without employing chemometric methods
[4].
Recently, great attention was paid to nanoparticle-based sensors due to the exceptional properties of nanomaterials, such as biocompatibility, conductivity and catalytic activity
[5][6][7]. With nanomaterials the problems associated with traditional organic dye-based colorimetric devices can be overcome. In recent years, the use of nanomaterial-based colorimetric sensors that can detect simply by the naked eye ultralow concentrations of target analytes has been widely studied
[8][9].
Noble metal nanoparticles, provided with distinctive physicochemical and optical properties, display higher extinction coefficients than dyes, allowing one to sense target analytes by color changes detectable by the naked eye
[10]. In addition, their unique localized surface plasmon resonance (LSPR) properties, associated with their characteristic colors, dispersion and aggregation status, make them ideal for sensitive colorimetric recognition of several chemical and biological analytes
[11].
In the past decades, huge progress has been made in developing metal nanoparticle-based colorimetric devices which sensing mechanism is based on interparticle distance-dependent principles
[12][13]. In these sensors, the nanoparticles’ aggregation and dispersion grade variation, induced by the analyte, changes the interparticle plasmon coupling properties detectable by LSPR spectral shifts and the evident solution color changes
[1]. From the first colorimetric device proposed by Mirkin et al.
[14], several LSPR-based colorimetric sensors were developed for different analytes, such as macromolecules
[15], living cells
[16] and metal ions
[17].
Unfortunately, this kind of sensor presents some disadvantages; for example, the colloidal nanoparticles’ auto-aggregation leads to false positive or false negative results and lower selectivity in some analyses. Moreover, the lack of cheap methods that could improve the performance of naked-eye-based sensors is another crucial point
[1].
The actual challenge is the growth of economic and trusted nanoparticle-based colorimetric sensors with great selectivity and sensitivity. The recent literature has reported new strategies for developing “non-aggregation” nanoparticle-based colorimetric sensors that rely on LSPR and will be potentially useful for detecting many analytes
[3][18]; they show good figures of merit if compared with the classical devices
[19].
Moreover, if compared with dye-based devices, metal nanoparticle-based sensors possess higher extinction coefficients, higher sensitivity, and better performances in colorimetric analyses
[1].
2. Ag Nanoparticles-Based Colorimetric Sensors
Au and Ag nanoparticles have been widely applied in colorimetric detection thanks to their enhanced LSPR optical property. Although AgNPs have been less used than AuNPs in colorimetric assays, very interesting works have been published regarding sensors based on aggregation, etching and growth of AgNPs. In this section, some examples of colorimetric sensors will be described
[20].
2.1. Colorimetric Sensors Based on AgNP Aggregation
2.1.1. Metal Ions Detection
An application of AgNPs for sensing Pb(II) ions after its complexation with dithizone was recently reported. Dithizone is a selective dye that forms stable complexes with lead and other divalent cations at pH around 6; it also has a sulfide group able to covalently bond metallic AgNPs. In this study, the Ag nanoparticles were added to a solution containing Pb(II)-dithizone complex. AgNPs aggregate after contact with Pb(II)-dithizone complex; consequently, their LSPR absorbance at 421 nm decreases; this variation is applied to detect lead(II) indirectly. The perspective of the method is its application to environmental and biological samples for a selective, sensitive, and rapid sensing of Pb
2+ [21].
Again for lead(II), Khan et al. proposed a low-cost and simple colorimetric sensor based on AgNPs obtained from the leaf extract of
Aconitum violaceum, a perennial plant species present in the Himalayan region of Pakistan, India, and Nepal. AgNPs have been proficiently synthesized thanks to the presence of a significant quantity of polyphenols in the extract. The so prepared AgNPs were used as a colorimetric sensor for Pb
2+. In fact, in the presence of lead(II), the nanoparticles evidenced a color change from yellow to red; this variation was monitored by UV–vis spectroscopy. Furthermore, the sensor showed good linearity being a concentration range of 0.5–25 μM and a detection limit of about 0.1 μM
[22].
Recently another low-cost and selective sensor for determining lead(II) in waters and wastewater samples was presented. In particular, a plasmonic colorimetric sensing strategy was applied using AgNPs modified with polyvinyl alcohol (PVA) and paper-based analytical devices (PADs). The procedure consisted of measuring by a UV-vis spectrophotometer the redshift of the LSPR absorption band of AgNPs/PVA in the visible region after adding Pb(II); moreover, the color intensity of PADs was recorded with a smartphone and processing the data by ImageJ software. The mechanism of color change due to the red shift from 410 nm to 550 nm resulted from the strong ion-dipole interaction of Pb(II) with AgNPs/PVA that perturb the stability of the nanoparticles, causing their aggregation. The calibration curve showed pretty good linearity from 20 to 1000 μgL
−1 and a limit of detection of 8 μgL
−1 by UV-vis spectrophotometric measurements; while a linear range from 50 to 1000 μgL
−1 and a LOD value of 20 μgL
−1 were obtained by PADs
[23].
An attractive work reported a selective and low-cost colorimetric sensor for detecting Pb
2+ and Hg
2+ using AgNPs obtained by a green synthesis mediated by a roots extract of Bistorta amplexicaulis. The color change of AgNPs from dark-brown to light yellow for Hg
2+ and to light brown for Pb
2+ is due to the complexation and aggregation of AgNPs in solution. Thus, this colorimetric sensor offers qualitative and quantitative information simply by naked-eye detection, without using expensive instruments; moreover, it is very sensitive for Pb
2+ and Hg
2+, presenting a detection limit of 0.2 µM and 0.8 µM for Pb
2+ and Hg
2+, respectively
[24].
Kumar et al. developed a photoinduced synthesis of very stable AgNPs using, as a reducing and stabilizing agent, an aqueous extract of
Murraya koenigii. The so synthesized nanoparticles were applied for the colorimetric detection of Hg
2+; a linear correlation between surface plasmon resonance (SPR) band intensity and increasing concentrations of Hg
2+ was observed (with a linear range from 50 nM to 500 μM). This behavior was explained considering an oxidation-reduction mechanism: by adding Hg
2+ into the AgNPs solution, the AgNPs are oxidized to Ag
+ and, accordingly, Hg
2+ ions are reduced to Hg
0. In fact, after the addition of Hg
2+, the dark brown color of the AgNPs solution disappeared and the SPR band intensity diminished with minor shifting towards the blue region
[25].
Dong et al. proposed a very sensitive and selective colorimetric method for determining Cd(II) by applying AgNPs capped with chalcone carboxylic acid. In the presence of Cd(II), the formation of a complex with CAA occurs, followed by particles aggregation and color variation from yellow to orange (i.e., the sorption peak shifts from 396 to 522 nm). The selective determination of Cd(II) was obtained since other cations such as Al(III), Fe(III), Cr(III), Co(II), Ca(II), Mg(II), Cu(II), Mn(II), Ni(II), Pb(II), Hg(II), and Zn(II), do not interfere significantly. The limit of detection is about 0.1 μM. This method was applied to determine Cd(II) in natural waters, and the results obtained agreed with those achieved by ICP-OES analysis
[26].
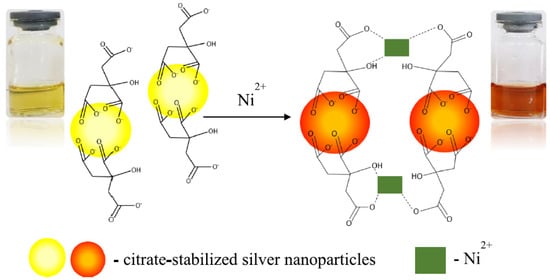
A simple colorimetric method using citrate-stabilized AgNPs for Ni(II) determination in waters was recently proposed. Sodium borohydride was used as a reducing agent and trisodium citrate as a stabilizer for the nanoparticle synthesis. Citrate-capped silver nanoparticles show a yellow color in solution provoked by the excitation of the local surface plasmon resonance band around 400 nm. Upon addition of Ni(II), almost immediately, a color change of the citrate-stabilized AgNPS from yellow to deep orange was observed; this variation was explained considering the particles aggregations due to the interaction between the Ni(II) ions with the carboxylic group present on the surface of the nanoparticles (see
Figure 1). A calibration curve linear from 0.7 to 1.6 mM Ni(II) concentration was obtained; the LOD and LOQ were 0.75 and 1.52 mM, respectively. Before the application of the method to real samples, interference studies were performed testing some representative cations such as Ag(I), Li(I), Ca(II), and Mg(II); the method showed excellent selectivity for Ni(II) compared to the other metal ions. Lastly, the method was applied for determining Ni(II) in synthetic solutions and tap waters, giving pretty good results
[27].
Figure 1. Colour change of the citrated capped AgNPs due to the particles aggregations caused by the interaction between the Ni(II) ions with the carboxylic group present on the surface of the nanoparticles. Reprinted with permission from
[27], under an Open Access Creative Common CC to Springer Nature.
An economical and eco-friendly method based on AgNPs for sensing Al(III) was presented. In particular, organic functional groups present in
Mentha arvensis plant extract are used as reductants and stabilizers in the synthesis of AgNPs, and also they provide a suitable active site for selective and sensitive Al(III) sensing. The presence of Al(III) in solution induced nanoparticles aggregation followed by a color change from yellow to reddish-brown. The modified AgNPs show high Al(III) sensitivity, and the calibration curve is linear from 200 to 1 nM metal ion concentration. This method was successfully applied for Al(III) detection in water samples where the cation was present at the nanomolar concentrations
[28].
Green synthesized AgNPs from
Moringa oleifera bark extract were prepared as sensitive Cu(II) sensors. The
Moringa oleifera plant contains amino acids, fatty acids, vitamins, and phenolics compounds; of these substances, glucosinolates are the most crucial functional groups thanks to their complexation properties towards metal cations. The change of the nanoparticles’ surface plasmon resonance (SPR) spectrum was studied by varying Cu(II) quantities, and a color change from brown to gray was verified. This variation is due to Cu(II) complexation by the benzyl glucosinolate present in
Moringa oleifera. A linear relationship absorbance vs. Cu(II) concentration in the range 10–90 μM was observed. The sensor’s selectivity towards Cu(II) was also verified since only small changes of the SPR band were registered in the presence of other different cations (i.e., chromium, mercury, zinc, and nickel)
[29].
2.1.2. Pharmaceutics, Biomolecules, and Environmental Contaminants Detection
A dual fluorescence-colorimetric assay for determining the antipsychotic drug olanzapine using Rhodamine B modified AgNPs was developed. The natural fluorescence of Rhodamine B was quenched upon reaction with AgNPs capped with citrate. Adding olanzapine to Rhodamine B-AgNPs, the analyte replaced the surface-bound Rhodamine B molecules, the aggregation of the nanoparticles occurred, corresponding fluorescence reappeared, and a colorimetric variation was observed. By fluorescence measurements, a linear concentration range of olanzapine from 0.05 to 10 μM was obtained; on the other hand, the colorimetric response is linear from 5.0 to 50 μM olanzapine concentration. The LOD values obtained by fluorescence and colorimetric measures were 0.013 μM and 1.25 μM, respectively. The good selectivity for olanzapine in the presence of other antipsychotic drugs, sugars, amino acids and cations, was demonstrated. Moreover, the assay was applied to determine olanzapine in pharmaceutical formulations and in a pharmacokinetic study of the drug in rats
[30].
2-Mercaptoethylamine, or simply cysteamine, is an aminothiol drug used in different clinical treatments, such as for cystinosis, Parkinson’s and Huntington’s neurodegenerative diseases and also applied as a neuroprotective agent. A recent work presents a colorimetric method for determining cysteamine based on the application of polyvinylpyrrolidone-capped AgNPs. Poly(vinylpyrrolidone) is a polymer widely employed in NP synthesis since it acts as a protective agent preventing nanoparticle aggregation. The sensing properties of PVP-AgNPs toward cysteamine were evaluated in aqueous solutions. In the absence of cysteamine, the suspension of the nanoparticle remained pale yellow colored with the characteristic SPR band at 395 nm. Increasing the cysteamine concentration from 0.2 to 2.0 μM, the peak intensity at 395 nm decreased, and a significant bathochromic shift from 395 to 436 nm was observed with the appearance of a new peak at around 560 nm. Simultaneously, a color change from pale yellow to purple appeared due to the nanoparticles’ aggregation upon the addition of cysteamine. The good selectivity of the sensor was tested considering several interfering compounds. Thanks to the promising results, the method was successfully applied to detect cysteamine in serum samples, suggesting its potential for biomedical analysis
[31].
In human physiology, urinary creatinine concentration is crucial since it is used to evaluate the renal function and diagnose possible diseases. In an interesting study, citrate-capped AgNPs were used as sensors for the colorimetric determination of creatinine in human urine. The detection mechanism bases on the creatinine-mediated aggregation of the citrate-capped silver nanoparticles at pH 12, which produces a detectable color change. This sensor showed a linear response from 0 to 4.2 µM creatinine concentration with a LOD of 53.4 nM. This method does not require further surface functionalization of the silver nanoparticles or sample pretreatment, so the advantages of simplicity, coupled with good sensitivity and selectivity, highlight its utility as a promising tool for creatinine monitoring in low-resource, point-of-care settings
[32].
The determination of arginine is very important since, in the human body, it leads to different effects such as modulation of immune function, insulin sensitivity, vascular tone, hormone secretion, and endothelial function. Arginine is also applied to detoxify the blood ammonia, and it is also involved in many pathways. A recent study reported a method for the quantification of arginine by using Ag NPs. In particular, Ag NPs conjugated with arginine reacting with an excess of Pb
2+ ions forming a complex; this reaction leads to color variation of the nanoparticles from brownish yellow to black. A liner relationship absorbance vs. concentration was observed from 1 nM to 1 mM arginine concentration; the LOD value is around 0.1 nM
[33].
A recent work presents an optical sensor for adenosine analysis in human urine. The sensor consists of AgNPs functionalized with an anti-adenosine aptamer as a sensing probe. The sensor response depended on the different stability of anti-adenosine aptamer-AgNPs in the presence of adenosine; in particular, the nanoparticles’ aggregation was gradually reduced by the increase of adenosine concentration. The method permitted the adenosine determination in a concentration range from 60 to 280 nM with a LOD of 21 nM. The reliability of the assay was established by detecting adenosine in urine samples of two lung cancer patients; the percentage of recoveries ranged from 98 to 107%
[34].
An interesting H
2S sensor based on gellan gum-stabilized AgNPs was developed for a real-time monitor of meat spoilage for intelligent packaging. The employment of AgNPs as the hydrogen sulfide sensor was due to the high reactivity of Ag with H
2S to form the salt Ag
2S, which caused notable LSPR changes of AgNPs. Moreover, the AgNPs were obtained using the linear exopolysaccharide gellan gum as a stabilizer and reducing agent for obtaining good nanoparticles’ stability during storage. Gellan gum is edible and has received U.S. FDA and EU (E418) approval for its use as gelling, suspending and stabilizing agent in various foods. The so prepared sensor can monitor meat spoilage in situ and in real-time since it can be part of an intelligent packaging system. In particular, a gellan gum-AgNPs hydrogel instead of the usual nanoparticle solution was used in meat packages. The hydrogel was prepared by adding agar into the nanoparticle solution. H
2S can permeate into the hydrogel, and its concentration increases with the exposure time until saturation. The sensor was applied to monitor chicken breast and silver carp spoilage with excellent results. Therefore, it can be used as a portable and low-cost H
2S sensor for accessible and instrument-free monitoring of meat spoilage in intelligent packaging
[35].
As well known, pesticides are substances employed to repel, kill, or control some forms of plant or animal life that are considered to be pests. Pesticides can cause short-term adverse health effects and chronic effects that can arise months or years after exposure. Some examples of chronic diseases are immunotoxicity, endocrine system disruption, cancers, congenital disabilities, and neurological pathologies. Therefore, new methods or tools are necessary to forecast the potential dangers of pesticides and thus reduce the adverse effects on the environment and human health
[36].
In this regard, a colorimetric method for sensitive and selective determination of the fungicide tricyclazole based on fluorescein-functionalized AgNPs was presented. The addition of tricyclazole to the functionalized nanoparticles induced the absorbance at 394 nm decrease and the evident color change from yellow to gray due to the aggregation of the nanoparticles. The selectivity of the assay was tested by analyzing the UV-vis spectrum of the functionalized nanoparticles in the presence of other pesticides (acetamiprid, bifenthrin, carbaryl, carbofuran, chlorpyrifos, clothianidin, 2,4-D, dichlorvos, dicofol, fenvalerate, glyphosate, glufosinateammonium, kresoxim-methyl, imidacloprid, methomyl, pencycuron, profenofos, propanil, thiodicarb, trichlorfon). The test results showed that the interferents did not cause the nanoparticles’ aggregation, and the only tricyclazole induced the color change and thus the variation of the UV-vis spectrum of the nanoparticles. The recoveries of the tricyclazole in spiked rice samples were from 94.6% to 103.0%, and a LOD of about 0.051 mg/L
[37].
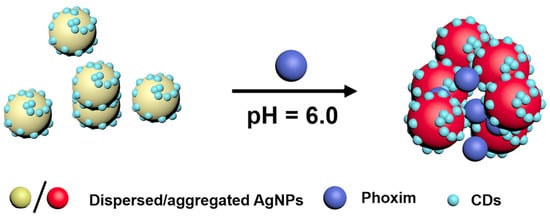
Phoxim (O,O diethyl-O-α-oximinophenyl cyanophosphorothioate) is an effective broad spectrum insecticide. An interesting study proposed a green synthesis of carbon dots-modified silver nanoparticles (CDs-modified AgNPs); NaBH
4 was used as a reducing agent and carbon dots as stabilizing agents. In solution at pH 6, the presence of phoxim induced the aggregation of the dispersed CDs-modified AgNPs and the consequent color change from yellow to red (see
Figure 2) due to the redshift of the SPR peak from 400 to 525 nm. The developed assay was applied to the phoxim detection in river waters, tap waters and apples with good recovery in the range of 87–110.0%. The LOD of the method is about 0.04 µM. Thanks to the sensitivity and selectivity performances, the sensor can be successfully applied to analyze environmental and food samples
[38].
Figure 2. Scheme of the detection mechanism of Phoxim by CDs-modified AgNPs. Reprinted with permission from
[38], copyright 2018 Elsevier B.V.
A one-pot microwave production of AgNPs from mature stem extract of the plant
Coscinium fenestratum and AgNO
3 was studied to develop a spectrophotometric sensor for the dithiocarbamate fungicide thiram. The stem plant extract also acted as a stabilizing agent of the nanoparticles. Thiram is a non-systemic fungicide commonly used in agriculture in the cultivation of fruit plants. The addition of thiram to a solution of the produced nanoparticles induced a change in the SPR peak at 423 nm. A decrease of the peak was observed in a hiram concentration range frotm 10 to 90 μM. This behavior was explained considering the intermolecular H-bonding interactions between thiram and the functional groups present in the capping agent. The sensor’s selectivity was evaluated considering as potential interferents the macro constituents of water samples; since the only few changes observed, the selectivity of the nanoparticles toward thiram was proved. A limit of detection of about 0.18 mg/L, lower than the limit value of 7 mg/L proposed by US-EPA, was obtained; so that this method can be promising to detect thiram in natural water and environmental samples
[39].
2.2. Colorimetric Sensors Based on AgNPs Anti-Aggregation
Although most colorimetric sensors are based on AgNPs aggregation, this approach faces some difficulties, such as the lack of stability and selectivity since many external factors can induce AgNPs aggregation. On the contrary, the anti-aggregation-based devices strategy could improve selectivity lowering the possibility of false-positive responses
[40].
Recently, a fluoride sensor based on the anti-aggregation of modified silver nanoparticles was developed. In particular, aggregated AgNPs were prepared using sulphanilic acid and catechol as stabilizing-reducing agents (SA-CAT-AgNPs). The presence of fluoride induced a color change of the aggregated nanoparticles solution from red to yellow, proportional to the fluoride concentration in the range from 1 to 40 μM with a LOD of 0.2 μM. The color variation was ascribed to the anti-aggregation of SA-CAT-AgNPs due to the adsorption of fluoride ions on the AgNPs surface and the simultaneous release of the SA-CAT capping agent. The sensor was applied for fluoride detection in Iranian spring waters. The results were not significantly different from those achieved by the standard potentiometric method with the ion-selective electrode, suggesting that the method can be promising for fluoride detection in natural waters
[40].
Chavada et al. proposed a colorimetric method for determining the concentration of deferiprone, an iron-chelating drug, based on AgNPs functionalized with pyrophosphate groups. The so obtained nanoparticles aggregated in the presence of iron(III) ions inducing the solution color change from yellow to red. After adding increasing quantities of deferiprone, the color of the nanoparticle solution turned yellow due to the anti-aggregation process, and the intensity of this variation was proportional to the deferiprone concentration. The method gave a linear response from 6.0–100 μM deferiprone concentration and a LOD of 0.28 μM. The selectivity was verified since no responses were obtained in the presence of many amino acids, saccharides, and inorganic ions. The method was applied for determining deferiprone in commercial drugs and a spiked human plasma demonstrating good accuracy, so this innovative and cheap assay can be promising for deferiprone dosage in biological fluids and pharmaceutics
[41].
Cheap and rapid assays for detecting bacteria are of huge importance. An interesting work proposed a colorimetric method for determining gram-negative bacteria (here,
E. coli was used as a model). It is based on the anti-aggregation of 4-mercaptophenylboronic acid-functionalized silver nanoparticles (MPBA-AgNPs). MPBA is one of the boronic acid derivatives; it could interact with
cis-diol groups present in the saccharide part of bacteria cells; so that it can be efficiently used as the recognition system in sensors devoted to bacterial detection. In the proposed method, the MPBA-AgNPs’ aggregation was inhibited by bacterial cells, and a consequent color change, from yellow to brown, was revelated. The dynamic range was between 5 × 10
4 cfu/mL and 10
7 cfu/mL
E. coli and the LOD of 9 × 10
3 cfu/mL was achieved. The sensor response is fast, requiring about 20 min. The feasibility of the assay in real samples analyses was verified by detecting E. coli in tap water spiked with different concentrations of bacteria (0.5 × 10
6, 10
6, 5 × 10
6 cfu/mL), obtaining pretty good recovering (from 93 to 107%) and standard errors below the 4%
[42].
2.3. Colorimetric Sensors Based on AgNPs Etching
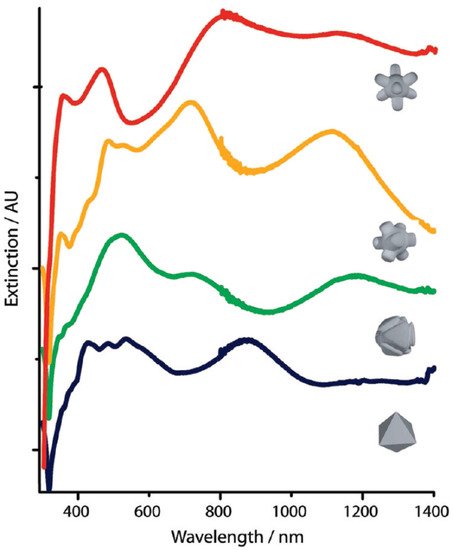
The study of several metal nanoparticle sizes, shapes, and clusters highlighted the importance of these parameters to customize the plasmonic characteristics of the nanomaterials. The development of chemical methods able to simultaneously control particle shape, size, and composition at the nanoscale level is fundamental. For example, a simple chemical etchant can be used to produce complex nanoparticles architectures.
Figure 3 shows the change of UV-vis-NIR spectra caused by the etching of octahedra AgNPs during the reaction upon adding particles to the etching solution. The color changed from tan to yellow, orange, purple and finally reddish-pink
[43].
Figure 3. UV-vis-NIR spectra of AgNPs during an etching process starting from octahedral-shaped particles. Etchant solution: H
2O
2/NH
4OH/CrO
3 in a 1:5:0.08 ratio. Reprinted with permission from
[43], copyright 2010, American Chemical Society.
The color change induced by the etching of AgNPs is one of the strategies adopted in developing colorimetric sensors. An interesting study presented a colorimetric assay for the detection of uric acid based on the suppression of oxidative etching of AgNPs by chloroauric acid
[44]. The etching occurs thanks to the oxidation of the Ag(0) to Ag(I) by HAuCl
4; consequently, Au(III) is reduced to Au(0) and deposits onto the silver surface, so forming Au-AgNPs with honeycomb shape evident for the color change from yellow to brown. In the presence of uric acid, the oxidization etching of AgNPs is inhibited; accordingly, the brown color disappears, and the absorbance peak is blue-shifted from 477 to 428 nm. This process has been exploited to develop the colorimetric sensor. A LOD of the method of about 30 pM, and a linear response range from 0.1 nM to 0.1 mM uric acid concentration, were reported. The trustworthiness of the method was successfully demonstrated by analyzing spiked serum samples since the results obtained were non significantly different from those achieved by a routine clinical method. Thanks to the excellent results, the sensors could be employed for medical diagnosis.
A similar approach was applied to develop a colorimetric sensor for captopril, an angiotensin-converting enzyme (ACE) inhibitor prescribed for the clinical treatment of hypertension, heart failure and for preventing kidney failure due to high blood pressure and diabetes
[45]. The sensing is based on tuning the localized surface plasmon resonance (LSPR) of triangular Ag nanoprisms by oxidative etching induced by Cl
− ions. In particular, a gradually blue shift from 660 to 420 nm occurred by increasing the chloride concentration, and consequently, a color change from blue to yellow arose; this is due to the transformation of the triangular-shaped nanoprisms in the smaller disc-shaped Ag nanoparticles. When captopril was added, it was bound on the Ag nanoprisms surface through an Ag-S bond involving the thiol group of the drug. Thereby captopril prevented the nanoprisms etching. Based on this process, a colorimetric assay was proposed for the quantitative detection of captopril, with a linear response range from 10 to 600 nM. A drawback is that biothiols can respond to the sensor and generate interference so that the method could be applied for quality control of captopril in pharmaceutical products, but it is not suitable for biological fluids analysis where interfering thiols may exist.
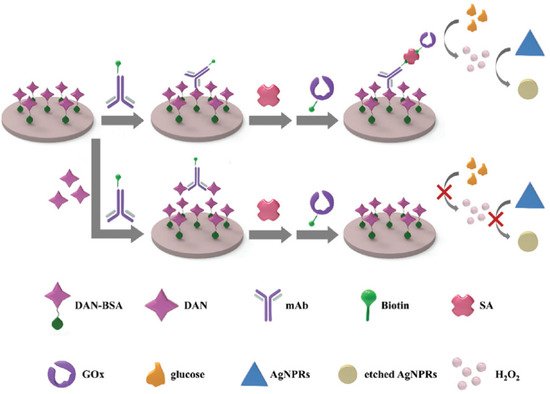
A plasmonic ELISA method based on the oxidative etching of Ag nanoprisms for the detection of danofloxacin, a fluoroquinolone antibiotic, was recently proposed
[46]. The method combines localized surface plasmon resonance (LSPR) of nanoparticles with the classical ELISA technique. It is based on glucose oxidase-catalyzed glucose degradation to etch the Ag nanoprisms, using an indirect competitive procedure. In
Figure 4, a scheme of the method is shown. In the absence of danofloxacin (DAN) molecules in the sample, the DAN monoclonal antibody linked to the biotin (biotin-mAb) can interact directly with the conjugate DAN/bovine serum albumin (DAN-BSA) coating the microplates. Then, the biotinylated GOx (biotin-GOx) was captured by biotin-mAb through the mediator streptavidin (SA). Successively, glucose was added, and reacting with biotin-Gox, produced H
2O
2, which caused the nanoprisms etching. The etching converted the nanoprisms to nanodisks with a corresponding solution color change from dark blue to colorless. On the contrary, in the presence of DAN, the fluoroquinolone molecules were competitively bound to biotin-mAb and inhibited its binding to the DAN-BSA coting. In this case, no H
2O
2 was produced; thus, the solution persisted blue since the nanoprisms retained their morphology. The calibration curve showed a linear range from 0.31 to 10 ng/mL of DAN with a LOD of about 0.2 ng/mL. The proposed method presented good selectivity, accuracy and precision for the danofloxacin determination, so this assay is promising for food safety analysis.
Figure 4. Scheme of the Ag nanoprisms-etching ELISA method for the detection of danofloxacin. Reprinted with permission from
[46], copyright 2012, Royal Society of Chemistry.
An interesting study demonstrated that AgNPs solutions could be used as a bifunctional probe, i.e., a colorimetric H
2O
2 chemosensor and a cholesterol biosensor
[47]. The sensor’s principle is based on the redox reaction between AgNPs and H
2O
2, which leads to the formation of Ag(0). The nanoparticles’ etching and the consequent color change from yellow to pinkish (or colorless depending on the amount of H
2O
2) were observed. A selective and sensitive H
2O
2 detection was obtained with LOD of about 3.5 µM. Afterward, a colorimetric biosensor was developed for cholesterol determination by combining AgNPs and the cholesterol oxidase enzyme (ChOx). ChOx oxidizes cholesterol into cholest-4-en-3-one and H
2O
2. Then, the H
2O
2 concentration, detected by the AgNPs etching, reflected the cholesterol level in the sample. This biosensor presented high selectivity and sensitivity for cholesterol detection with a LOD of about 40 µM; it could be efficiently applied to dose cholesterol in serum samples, so the bifunctional probe was promising for determining both oxygen peroxide and cholesterol in biological samples, and it is suitable for clinical and medical applications.
2.4. Colorimetric Sensors Based on AgNPs Anti-Etching
Some new recent works have proposed colorimetric assays based on anti-etching of silver nanoobjects. An interesting example is that proposed by Fang et al. The authors described a colorimetric method for determining dopamine in serum, based on the protection of the silver nanoprisms etching induced by Cl
− ions when adding dopamine. Indeed, in the absence of dopamine, chloride ions tend to attach to the edges and angles of the Ag nanoprisms forming Ag-Cl bounds and etching the nanoprisms in nanodisks followed by a color change from blue to yellow. In the presence of dopamine, the catechol group of this molecule was quickly adsorbed onto the silver nanoprisms’ surface; so dopamine acted as a protective agent toward the nanoparticles’ etching induced by Cl
−. The anti-etching process induced a color variation proportional to the dopamine concentration in the range between 0.5 to 100 nM, The LOD of the method was about 0.16 nM. The applicability of the assay was verified by analyzing human serum samples fortified with dopamine, obtaining good recovery, ranging from 97 to 105%; these results, along with the rapidity, ease of performance and selectivity, make the method promising for biological analyses
[48].
Another work, inspired by the previously described one, proposed a colorimetric method for Hg(II) ions detection based on anti-etching of triangular silver nanoplates. In the absence of Hg(II), the triangular nanoplates are etched to nanodiscs in the presence of chloride ions, followed by a color change from blue to yellow. In the presence of Hg(II) ions, an amalgam Ag/Hg was formed due to the adsorption and the consequent reduction of mercury ions; an evident color change from yellow to brown, purple, and blu was obtained proportionally to the amount of Hg(II) in solution. The method showed a linear response from 5 nM to 100 nM Hg(II) concentrations with a LOD of about 0.35 nM. The method’s validation was achieved by analyzing certified material and river water samples, comparing the obtained results with those achieved by classical chromatography methods
[49].
Recently a colorimetric assay for detecting biothiols was presented. It is based on the anti-etching of silver nanoprisms. In particular, a double function of biothiols as anti-etching and aggregating agents for the nanoprisms was observed. At pH higher than 5 and in the absence of biothiols, silver nanoprisms can be etched by chloride ions to gave spherical AgNPs and a correspondent color change from purple/blue to yellow. Adding biothiols to the nanoobject solutions, the Ag-S interaction prevents the nanoparticles’ etching, and the color return purple/blue. It was also demonstrated that the individual biothiols homocysteine, cysteine, and glutathione, also exerted an aggregation effect that can be tuned by changing the solution pH. The method was applied to determine the total amount of biothiols in human serum samples; the method’s accuracy and rapidity, convenience, and direct determination make the assay promising for biological analysis
[50].
2.5. Colorimetric Sensors Based on AgNP Growth
Ag
+ can be used as precursors for AgNPs in growth-based colorimetric sensors; seeds are also sometimes used to grow nanoparticles. For example, a fluorometric method for determining sulfadiazine (a common sulfonamide antibacterial widely used in veterinary medicine to treat infectious diseases) based on the growth of AgNPs on graphene quantum dots was recently proposed
[51]. Ag-NPs on graphene quantum dots (AgNPs-GQDs) were synthesized by reduction of silver nitrate with sodium borohydride in the presence of the graphene quantum dots. The quenching of the blue fluorescence of the GQDs (emission maximum peak at 470 nm) was caused by the growth of the nanoparticle on the quantum dots’ surface. The addition of sulfadiazine and its interaction with AgNPs restored the fluorescence. The fluorescence increased linearly by sulfadiazine additions in a concentration range from 0.04 to 22.0 μM. LOD and LOQ were found to be 10 and 37 nmol/L sulfadiazine, respectively. Since the good selectivity, rapidity, and high sensitivity, the method could be promising for determining sulfadiazine in biofluids and complicated matrices.
Another interesting and very recent work presented a colorimetric sensor for
p-aminophenol based on seed-mediated growth of silver nanoparticles
[52].
p-Aminophenol is an organic compound classified as nephrotoxic and teratogenic. It is a building block used in several organic and industrial syntheses, and it is the final intermediate in the industrial preparation of paracetamol.
p-Aminophenol in urine is one of the markers for evaluating the exposure of carcinogenic aniline compounds, and it is also present as the main impurity in paracetamol-based drugs. Thus, accurate methods for quantifying
p-aminophenol in urine samples and paracetamol-based drugs is of paramount interest. This study proposed a colorimetric sensor based on the capability of
p-aminophenol to reduce Ag
+ ions, thus enhancing the growth of AgNPs. A solution containing AgNPs as seeds, AgNO
3 and a small amount of NaBH
4 was prepared for obtaining the AgNPs–Ag
+ mixture. After adding
p-aminophenol to the AgNPs–Ag
+ mixture, further growth of AgNPs was observed by the color change from yellow to orange. The sensor provides good sensitivity and selectivity, with LOD of 15 and 0.32 μM by naked eyes and UV-vis spectrometry, respectively. The good precision and high recovery % were demonstrated by analyzing different human urine samples and the drug Panadol.
3. Au Nanoparticle-Based Colorimetric Sensors
Gold nanoparticles (AuNPs) can be efficiently applied to develop colorimetric sensors since they can be simply functionalized, showing different colors depending on their shape, size, and aggregation state. In the last decade, various sensors were developed, taking advantage of the nanoparticle’s color changes in the presence of different analytes.
Recently, novel colorimetric sensors based on the changes of nanoparticles morphology have been developed. In particular, the evolution of AuNPs’ shape can be due to etching or growth. So, the changes in the nanoparticles’ size, shape and composition, induced by the analytes, are followed by a shift in the SPR band, and a color change appears
[53].
In this section, recent applications of AuNPs in colorimetric sensors are discussed.
3.1. Colorimetric Sensors Based on AuNPs Aggregation
Aggregation of AuNPs promoted by analytes is the key point to prepare these sensors. The interaction between analytes and nanoparticles can be obtained by functionalization of the AuNP
s with specific ligands. Typical examples of colorimetric sensors based on AuNP aggregation are those for metal ions; they have been applied in different fields such as environmental, clinical and food analysis. To this end, chelating ligands containing carboxylic, hydroxyl and amino groups are linked to the nanoparticles’ surface. The so functionalized AuNPs, in the presence of metal ions, aggregate thanks to the formation of metal ion/ligands complexes, with a resulting color change from red to purple
[54].
For example, AuNPs functionalized by N-lauroyltyramine (NLTA) were prepared for Al(III) sensing. The presence of Al(III) induced the nanoparticles’ aggregation with the consequent color change from pink to purple. The selectivity was tested by analyzing several other metal ions. Among these metal ions, the NLTA-AuNPs aggregation was induced only after the addition of Al(III). The detection limit reported is about 1.15 µM, and the dose/response curve is linear from 0 to 12 µM. The sensor was applied to Al(III) detection in-ground and sewage waters, giving good recovery
[53].
As well-known, arsenic is a very toxic element responsible for several acute and chronic poisoning in humans. AuNPs based colorimetric sensors for As(III) are generally based on the strong binding affinity of the cation to sulfur-containing compounds. Based on this property, Kalluri et al. functionalized AuNPs with glutathione, dithiothreitol, and cysteine for obtaining colorimetric sensors for As(III). The detection limit of the sensors was 5 ppb, 20 ppb, and 25 ppb for dithiothreitol, glutathione, and cysteine-conjugated NPs, respectively. The lower detection limit obtained for AuNPs functionalized with dithiothreitol was probably due to the As-S bond; conversely, in the other two sensors, the bonds As-O were expected due to the lack of free SH groups
[55].
Besides metal ions, colorimetric sensors based on AuNP aggregation were also developed for some organic molecules. For example, simultaneous recognition of amino acids by 4-aminonicotinic acid-functionalized AuNPs was proposed
[56]. The presence of arginine, histidine, methionine and tryptophan, induced the nanoparticle’s aggregation through electrostatic interactions and hydrogen bonding.
Specific and sensitive DNA detection is crucial for clinical diagnostics (for example, diagnosing genetic diseases, biodefense and RNA profiling). Interesting studies proposed the design of thiolated oligonucleotide modified AuNPs sensors. These probes possess complementary structures that capture target DNA, resulting in the hybridization of DNA into a double helix, self-assembly of AuNPs and a consequent change in the solution color
[57].
3.2. Colorimetric Sensors Based on AuNP Anti-Aggregation
Nowadays, sensing based on AuNP anti-aggregation strategies has also been of interest. In these devices, generally, a reagent first provokes the aggregation of AuNPs. Then the addition of an analyte with a high affinity to the aggregating reagent deactivates the nanoparticles’ aggregation in a degree proportional to its concentration. In this way, the process can be used for the analyte determination
[53].
For example, Chen et al. developed a colorimetric sensor for Hg(II) ions based on anti-aggregation of AnNPs functionalized with thiocyanuric acid. The assay is based on the high affinity of mercury ions towards thiols. In the presence of thiocyanuric acid, gold nanoparticles aggregate thanks to the interaction between the Au surface and the thiol group inducing a color variation from red to blue. After adding Hg(II), the thiocyanuric acid is removed from the nanoparticle surface since mercury ions are more active towards thiols than gold; consequently, the anti-aggregation of AuNPs occurred, and the solution color turned from blue to red. The practical application of the method was evaluated by applying it to trace Hg(II) determination in certified reference material and natural waters, obtaining good recovery. Thanks to these results, the method can also be useful for Hg(II) determination at low concentrations below 1 nM
[58].
Another more recent example proposed by Huang et al. relates the development of a colorimetric sensor for methionine in serum and urine, based on the anti-aggregation of AuNPs in the presence of melamine. Citrate capped AuNPs can aggregate in the presence of melamine due to the ligand exchange between the negatively charged citrate ions and the positively charged amino groups of melamine, inducing a solution color change from wine red to blue. Nevertheless, in the presence of methionine, the gold nanoparticles are more disposed to interact with it through Au-S and Au-N bonds, inducing the nanoparticles’ anti-aggregation. To evaluate the sensor’s practicability, analyses of human serum and urine samples spiked with methionine were performed. Thanks to the good recovery obtained (from 95 to 105%), the method could be promising for methionine determination in biological fluids
[59].
3.3. Colorimetric Sensors Based on AuNPs Etching
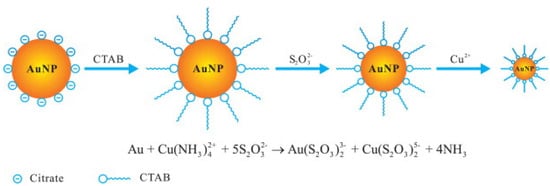
Analytes with high redox potential or acting as complexing ligands of noble metal ions can be detected by AuNPs etching. For example, a colorimetric Cu(II) sensor based on AuNPs etching was developed. Firstly, AuNPs (capped with citrate) were stabilized by hexadecyltrimethylammonium bromide (CTAB) in an ammonium buffer solution. In the presence of S2O32− solution, the formation of the complex Au(S2O3)23− occurred, and consequently, the stabilized gold nanoparticles were partly dissolved, with a decrease of the SPR absorption. After the addition of Cu(II)) the dissolving process accelerated, and the solution color of the solution disappeared rapidly. Figure 5 shows a scheme of the process.
Figure 5. Scheme of the Cu(II) sensing based on the catalytic etching of AuNPs. Reprinted with permission from
[60], copyright 2013 Elsevier B.V.
The color change induced by adding Cu
2+ in concentration at least 40 nM can be easily observed by naked eyes so that the sensor can be promising for fast on-site analysis
[60].
Based on the same principle, a cyanide sensor was proposed using gold nanorods (AuNRs). AuNRs were etched by cyanide at the transverse faces, leading to a variation of the AuNRs aspect (they were transformed from nanorods into nanosphere), resulting in a blue-shifted SPR absorption and color change from blue to pink
[61].
3.4. Colorimetric Sensors Based on AuNP Anti-Etching
Some analytes can decrease or impede the etching of AuNPs, so the nanoparticles keep their initial morphology; this process can be useful to develop colorimetric sensors.
An interesting example was recently proposed by Wang et al. who developed a colorimetric sensor for sulfide ions using triangular Au nanoplates. In particular, the gold triangular nanoplates can be etched and transformed into nanospheres in the presence of triiodide ions produced by the reaction between Cu(II) and iodide. After adding sulfide ions, Cu(II) reacted preferentially with S
2− inhibiting the nanoplates etching; consequently, a redshift of the LSPR peak, proportional to the sulfide concentration, was observed. The sensor showed a linear response in the sulfide concentration range from 0.02 to 1.5 μM, with a LOD of about 16 nM. The good results obtained by applying the method to natural water samples make it promising for sulfide analyses of environmental matrices
[62].
The same authors also proposed a colorimetric assay for the sequential determination of Cu(II) and Hg(II) still based on etching/anti-etching of gold nanoplates. Firstly Au nanoplates were etched in the presence of I3-obtained by a redox reaction between Cu(II) and an excess of iodide; the shape of the nanoplates changed from triangles to spheres, and a consequent color variation from blue to pink, of a degree proportional to Cu(II) concentration, can be observed. Then, after adding Hg(II) ions, the etching was inhibited since I
− was consumed for HgI
2 salt formation. Thus the nanoplates’ shape has returned triangular, and the solution color varied from pink to blue, with an anti-etching degree proportional to Hg(II) concentration. The linearity of the dose-response curve was verified in the range 10 nM–1.5 μM for Cu(II) and 20 nM–3 μM for Hg(II). The LODs were reported equal to 19 nM and 9 nM, respectively, for Cu(II) and Hg(II). The method was applied for determining Cu(II) and Hg(II) in natural waters and food samples. Since the pretty good results, this colorimetric assay seems promising for a quick (the whole analysis has a duration of about 15 min) and sensible sequential determination of Cu(II) and Hg(II)
[63].
3.5. Colorimetric Sensors Based on AuNP Growth
Some analytes with high reduction potential can react with AuCl
4− to form nanoparticles so that when AuNPs act as seeds in the reaction between AuCl
4− and a reductant, the formation of gold nanoshells on the surface of the nanoparticles induces a variation in the SPR spectrum. Gold nanoparticles, nanocages and nanorods were applied as seeds for the formation of nanoshells; during this process, the variation of the shape and composition of nanoparticles induces the change in the SPR spectrum and the consequent color change of the solution. Sensors based on this phenomenon were developed to detect, for example, amino acids,
p-aminophenol, catecholamine neurotransmitters and ellagic acid
[64][65][66][67].