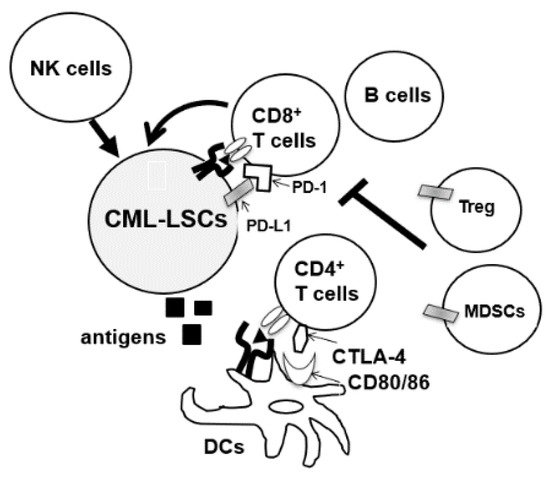
Dasatinib is known to induce the expansion of large granular lymphocytes (LGLs)
[19]. Kreutzman et al. reported the development of dasatinib-associated lymphocytosis at an average of three months after the start of treatment and its persistence throughout the therapy. The expanded lymphocytes contained both CD3
+CD8
+ effector memory T-cells and CD3
−CD16
+CD56
+ NK cells
[20]. Importantly, the median progression-free survival or overall survival was superior in patients with increased LGL expansion compared to those without
[21]. Dasatinib is also known to affect Tregs and NK cells. In a prospective phase II clinical trial, D-first study, peripheral blood of 52 patients with newly diagnosed CM-CP was observed for a minimum of 36 months after starting daily administration of 100 mg dasatinib. Treg decreased with dasatinib therapy, and the proportion of Tregs at 12 months of dasatinib treatment was associated with the achievement of deep molecular response (DMR). Treg inhibition was also correlated with an increase in CTL counts, NK cell count, and NK cell differentiation
[22]. The effects on subsets of NK cells have also been analyzed in several studies. Ishiyama et al. recently reported that CD56
− NK cells with PD-1 expression were induced by chronic activation through CMV reactivation in CML or Ph + ALL patients during dasatinib therapy
[23]. Interestingly, the expansion of CD56
− NK cells was associated with a higher rate of DMR, and Wei et al. also reported that dasatinib increased the percentage of Th1 and CD8 T cells and decreased Treg cell levels in the peripheral blood of CML patients who responded to therapy
[24]. Overall, dasatinib seems to have the potential to enhance both innate and adaptive immunity to eradicate CML-LSCs in some patients. Clinical trials (ClinicalTrials.gov Identifier: NCT04991532) are also ongoing in China to assess the effects of dasatinib on NK cells and T cells of CML-CP patients to compare the immunomodulatory effects of TKIs in clinical settings.