1000/1000
Hot
Most Recent

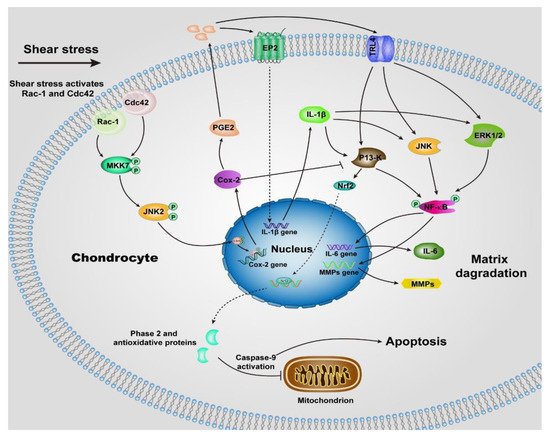
The remarkable lubrication properties of normal articular cartilage play an essential role in daily life, providing almost frictionless movements of joints. Alterations of cartilage surface or degradation of biomacromolecules within synovial fluid increase the wear and tear of the cartilage and hence determining the onset of the most common joint disease, osteoarthritis (OA).
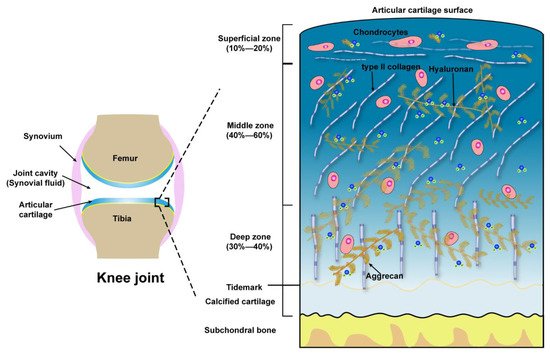
| Zones | Extracellular Molecules | Biomechanical Properties | References |
|---|---|---|---|
| Superficial zone (10 to 20%) |
Outer of Surface Lubricin, HA, Phospholipids, COMP | Boundary lubrication, chondroprotection |
[35][36][37] |
| Below outer of Surface Type II collagen aggrecans, HA |
Resist shear stress, bear ~20% load; Maintain tensile strength; As a barrier to fluid flow during loading; Subject to maximum strain; Contribute to elasticity and resiliency via interacting with collagen |
[38][39][40] | |
| Middle zone (40 to 60%) |
Upper ~1/3rd Collagen type II, other collagens, aggrecans, HA |
Transit shear and compression stresses; Exhibit high deformation during loading; Resist compression; Contribute to elasticity and resiliency to compression via interacting with collagen | [38][39][40][41] |
| Lower ~2/3rd Thick collagen type II, other collagens, aggrecan, HA, GAGs |
Compared with upper 1/3rd of the middle zone: Decrease tensile strength; Provide higher resistance to compression during loading | [42][43][44] | |
| Deep zone (30 to 40%) |
Thickest collagen type II, other collagens, aggrecans, HA, high GAGs | Relative to the middle zone: Further decreased tensile strength; Provide highest resistance to compression during loading | [42][43][44] |