3. Current Insights
The relationship between Aβ generation and degradation determines the cerebral Aβ accumulation, which is one of the major histopathological hallmarks of AD
[22][23]. While it is well established that Aβ production is strongly affected by lipids and FAs
[1][2], less is known about the impact of the lipid environment on Aβ degradation. Therefore, in the present study, we analyzed the effect of FA acyl chain length on the enzymatic degradation of the peptide.
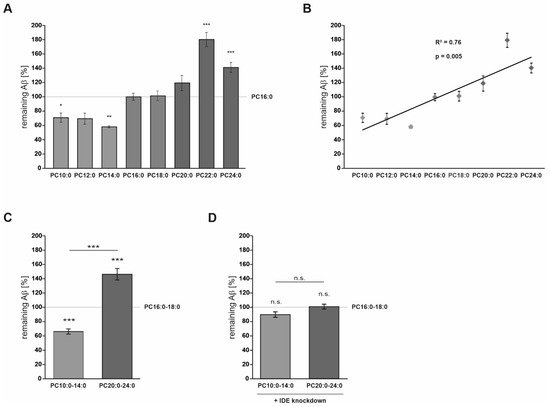
Neuro2a cells were exposed to saturated FAs with an acyl chain length ranging from 10 to 24 C-atoms before total Aβ degradation was measured. PC represents the most abundant phospholipid in mammalian cellular membranes
[24], palmitic acid (16:0) and stearic acid (18:0) are the major SFAs in human brain tissue
[13]. Correspondingly, FAs were applied as PCs containing identical FAs in the sn1- and sn2-position, and PC16:0 or PC16:0–18:0 (for the grouped evaluation) were chosen as controls. This experimental setup enabled us to analyze the impact of the FA acyl chain length regardless of possible effects of the choline headgroup or the glycerophosphoric acid. Liposomes containing the used phospholipids are efficiently taken up by cells. This results in the incorporation of the supplemented PCs into cellular membranes, probably affecting, e.g., membrane fluidity and structure, or their phospholipase A-dependent hydrolysis into lysolipids and free FAs
[25].
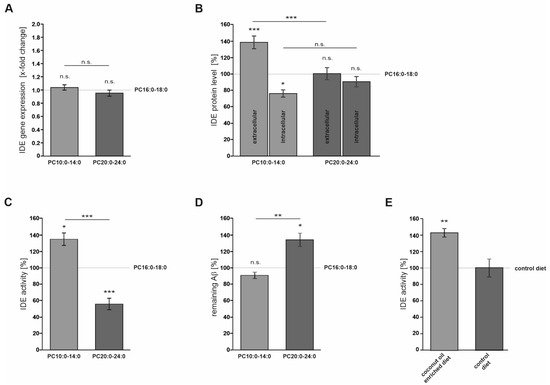
In Neuro2a cells, PCs containing MCFAs and shorter-chained FAs (PC10:0–14:0) significantly stimulated the degradation of exogenous human Aβ40 peptides conducted by IDE, one of the major Aβ-degrading proteases
[20][26][27]. The elevated Aβ degradation in the presence of PC10:0–14:0 was accompanied by changes in IDE sorting and a direct stimulating effect on IDE activity (
Figure 3). PC10:0–14:0 was found to specifically increase the release of IDE into the extracellular space, a process which occurs at least partially in association with exosomes
[19][28]. IDE exosomally released by Neuro2a has been reported to be proteolytically active as the inhibition of exosome release leads to increased endogenous Aβ levels in the cell culture supernatant
[19]. Considering that both exosome release in general and exosome-associated IDE secretion are known to be strongly affected by various lipids
[28][14][29][30][31][32], one can assume that saturated MCFAs and shorter-chained FAs stimulate the exosomal IDE secretion into the extracellular compartment. This might lead to the elevated degradation of synthetic human Aβ40 peptides added to the medium of cells treated with PC10:0–14:0, which could be further strengthened by the increased IDE activity in presence of these PC species. In this context, a recent publication of Song et al. should be mentioned which reports that IDE is not secreted from cultured cells, but rather released nonspecifically as a consequence of reduced membrane integrity. In this study, only ~1% of total cellular IDE was released from HEK-293 and BV-2 cells. In the conditioned medium of intact Neuro2a cells, the enzyme was almost undetectable, even after concentrating the samples 10-fold
[33]. In contrast, we and others
[19] found measurable amounts of IDE in the cell culture supernatant of this cell line. Possible reasons for these divergent results include differences in the used growth medium, the cell to medium ratios, the methods for the concentration and detection of IDE as well as clonal heterogeneity. Further, Song et al. found LDH and two other measured cytosolic markers (glyceraldehyde dehydrogenase (GAPDH) and pitrilysin) to be released at the same relative levels as IDE from HEK-293 and BV-2 cells treated with lovastatin, implying that IDE is released nonspecifically by lysed cells
[33]. However, unlike lovastatin, PC10:0–14:0 seems to specifically stimulate the secretion of IDE since it did not affect the release of SEAP, a marker for the constitutive secretory pathway
[34]. Additionally, two established indicators for cytolysis, the activity of LDH in the conditioned cell culture medium and the cellular uptake of PI
[35][36], were unaltered by the different phospholipids. Altogether, these data make a disruption of plasma membrane integrity very unlikely as the cause for the increased IDE release from cells treated with PC10:0–14:0. This is in line with other studies reporting a release of IDE into the cell culture supernatant in the absence of detectable LDH
[20][19]. The reason for these differences is unclear but could be based on differences in the cell lines used and the cytotoxic potential of the applied stimulants.
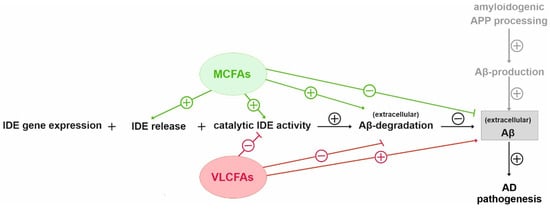
Figure 3. Schematic overview of the proposed effects of MCFAs and VLCFAs on IDE dependent Aβ degradation. The increased Aβ degradation in the presence of PC10:0–14:0 seemed to be based on changes in IDE sorting and a direct stimulating effect on IDE activity. In contrast, PC20:0–24:0 decreased the IDE-dependent Aβ degradation, probably by directly inhibiting the catalytic activity of the enzyme.
On the other hand, the saturated VLFAs contained in PC20:0–24:0 significantly reduced the IDE-dependent Aβ degradation, probably by directly inhibiting the catalytic activity of the enzyme (
Figure 3). In line with these data, an in vitro-inhibition of IDE-dependent insulin degradation by long-chain free FAs (C16–C20) with IC50-values ranging from 10 to 50 µM has been reported in the study by Hamel et al., mentioned above
[37]. A direct interaction between FAs and IDE is further supported by the suspected “cytosolic fatty-acid binding proteins signature” within the enzyme and our previous observation that different FAs distinctly bind to recombinant IDE
[37][14]. Altogether, our cell culture data indicate that saturated MCFAs and shorter-chained FAs are more beneficial than saturated longer-chained FAs and VLCFAs with respect to IDE-dependent Aβ degradation.
An increased IDE activity was also observed in the serum of mice upon dietary supplementation of MCFAs in the form of coconut oil. We would like to point out that the effects obtained with transgenic mice fed with coconut oil are comparable to the effects observed in cell culture. By utilizing an IDE knock-down, we could confirm that the major effect of coconut oil is linked to the Aβ degrading enzyme IDE. Due to limitation of samples size in mice, this confirmation which would be ideally done by a specific inhibitor of IDE (e.g., 6bK) was not possible. Therefore, we cannot rule out that, in vivo, other proteases besides IDE are affected by coconut oil, which is a potential caveat in our study. In addition, we suggest further studies investigating whether mutations in the “cytosolic fatty acid binding protein signature” would reinforce the notion that MCFAs regulate IDE. In order to address this question, further research is warranted.