1. Introduction
1.1. Breast, Colon, Lung, Prostate and Leukemia: The Deadliest Cancers
Lung, breast, prostate, and colon cancers have the highest overall incidence and represent 36.4% of the total diagnosis according to GLOBOCAN 2018, a report that gathers data from the International Agency for Research on Cancer of the World Health Organization
[1]. Besides their high occurrence, these types of cancer add up to a mortality rate that reaches 49.2% of the diagnosed cases. In addition, blood malignancies count with a rate of 6.5%, an incidence particularly worrying as it is the highest in children and young adults.
In the last five years international scientific production related to new basic and translational discoveries on cancer has grown by 5% annually. Lung, colon, breast, prostate, and leukemia cancers account for 51% of scientific publications on cancer in this period (Web of Science, Clarivate Analytics, Philadelphia, PA, USA). Many papers address the association between gene regulation and cancer progression or metastasis, still representing the main approach in cancer research.
1.1.1. Lung Cancer
Survival is the foremost concern in lung cancer due to its progressive metastasis and resistance to therapy and, furthermore, the molecular hallmarks of its malignant properties are still insufficiently known. Recently, a 17-gene panel involving critical cellular processes such as hypoxia-mediated epithelial-mesenchymal transition (EMT) or epigenetic modifications was identified as a potential biomarker tool for prediction of metastasis and prognosis in patients affected by non-small-cell lung cancer (NSCLC)
[2]. Other example of the last advances in this field is the detection of the scaffold WT1-interacting protein (WTIP) as a novel tumor suppressor down-modulated in NSCLC. Lower levels of these proteins significantly correlate with poor prognosis as a result of higher rates of cell proliferation and tumorigenesis
[3]. Additionally, increasing findings in immunotherapy open expectations for the improvement of the prognosis of lung cancer patients
[4].
1.1.2. Breast Cancer
Regarding breast cancer, tumor heterogeneity makes patient classification according to their risk of metastatic relapse essential in order to guide decisions in adjuvant chemotherapy. However, in the era of personalized medicine, genomics alone does not provide enough knowledge to apply precision treatments to specific tumors. Therefore, complementary multi-omic studies are emerging as the most promising approaches addressing breast cancer complexity
[5]. For instance, a quantitative proteotyping approach based on sequential windowed acquisition of all theoretical fragment ion spectra mass spectrometry (SWATH-MS) has been proposed to establish key proteins for breast tumor classification
[6].
1.1.3. Prostate Cancer
The application of new technologies now allows progressing in the understanding of the molecular basis of prostate cancer pathogenesis. Microarray-based transcriptomic analyses and next generation sequencing technology are examples of powerful tools for studying gene expression and changes in gene structure at the transcript level. Recent publications described new mechanisms regarding tumor suppressors in this type of tumors
[7] and new potential therapeutic targets
[8]. Also, these technologies played a critical role in recent studies indicating specific gene fusions, present in at least 50% of prostate tumors, as key modulators of gene expression promoting tumor growth and progression in prostate cancer
[9].
1.1.4. Colorectal Cancer
Deregulated cellular energetics is another of the hallmarks of cancer. In this sense, cooperative lipid metabolism-related genes involved in colorectal cancer progression have been recently identified. The acyl-CoA synthetase/stearoyl-CoA desaturase (ACSL/SCD) lipid network fuels migratory and invasive properties through EMT induction and is associated with an increased risk of relapse in colorectal cancer patients
[10]. Other molecular mechanisms associated with the refractory nature of cancer point to cancer stem cells (CSCs). In this regard, it has been recently published that proliferation of CSC-enriched colon spheroids is dependent on mTORC1 kinase, which is activated by reactive oxygen species (ROS) produced by an NADPH oxidase
[11]. Furthermore, the potential correlation between gene polymorphisms and colon cancer progression continues being studied and validated in order to identify prognostic indicators supported by mechanisms such as the increased invasiveness of tumor cells through the expression of genes involved in the activation of fatty acids through conversion to acyl-CoA
[12], or the regulation of COX2 expression and cell apoptosis
[13]. On the other hand, it has been recently discovered that microbiota plays an important role in colorectal carcinogenesis. This opens up new opportunities for using microbiota profiling information in colorectal cancer prevention, diagnosis, and therapy
[14].
1.1.5. Blood Malignancies
Recent advances in leukemia genomics and epigenomics have facilitated the study of clonal populations and their genetic-epigenetic evolution, changing the classic view of leukemia into a complex heterogeneous disease aggravated by clonal evolution
[15][16]. These findings allow a better understanding of the mechanisms involved in leukemia transformation and therapy resistance and reinforce precision medicine as the most promising approach to leukemia treatment. The complexity of this disease, together with the high number of patients showing chemotherapy resistance, evidences the urgent need of more efficient therapies. In the last years, new therapeutic approaches are being investigated, as for instance monoclonal antibodies-based treatments
[17] or therapies based in macrophage targeting
[18].
1.2. Cancer Relapse
Cancer relapse worsens the prognosis of patients and is a factor that contributes significantly to mortality. However, only 2% of cancer publications in the last five years deal specifically with relapse (Web of Science, using databases including MEDLINE). Cancer recurrence involves many biological interactions, such as genetic, transcription, environmental, endocrine signaling, and metabolism. These interactions add another layer of complexity in the understanding of cancer recurrence and metastasis, delaying progress in therapeutic opportunities
[19]. Lung cancer and leukemia mortality rates are mainly due to their higher recurrence rates, compared to breast, colon, and prostate, for which surgical resection of the tumor combined with adjuvant treatments accomplish higher survival rates
[20].
In essence, the recent discoveries in relation to drug resistance and previously unknown molecular mechanisms associated with metastasis and recurrence demand alternative therapeutic strategies, among which metabolism could emerge as a new cancer therapy support. Although cancer relapse is due to a multitude of genetic mutations and biochemical processes, advances in genomics, metabolomics, and proteomics allow better understanding of the metabolic diversity due to genetics and microbiome variation, as well as a detailed classification of tumors, which provide precision medicine with individual treatments. Likewise, the best understanding of the metabolic variation allows us to know the interactions between nutrients, metabolism, microbiota, and related genes, facilitating the development of adjuvant cancer therapies based on precision nutrition strategies
[21].
1.3. Precision Nutrition and Cancer Therapy
Over the years, numerous epidemiological studies have been carried out to link the diet with cancer, either from a preventive approach or by associating the consumption of certain food products with tumor generation and growth. However, in parallel to the development of precision therapies in medicine, precision nutrition is an emerging science that relies on well-established factors such as genetic and epigenetic variation
[22] and the microbiome
[23]. It has recently been shown that the treatment of human cell lines with different bioactive foodstuffs influences their physiological attributes depending on their ability to influence the expression of different genes
[24]. The possibility of using nutritional therapies against cancer, as a complementary medicine, is internationally accepted due to its advantages of less toxicity and better acceptance by patients
[25]. In the case of breast cancer, complementary phytochemical therapy in adjuvant treatments has been proposed both with preventive effects and during conventional treatments after diagnosis
[26], concluding that nutritional strategies can be effective for prevention of relapse
[27].
Epidemiological studies triggered further research in terms of molecular mechanisms, which have significantly improved the effectiveness of phytotherapy, entering the context of precision nutrition
[28]. Recent studies on the association between prevention, treatment, and recurrence of cancer suggest the benefit of investigating the link between specific food components and certain health outcomes
[29]. Since very specific therapeutic targets must be reached, precision nutrition must be based on individual foodstuffs with well-established mechanisms of action at the molecular level in terms of gene expression modulation and signaling pathways involved in proliferation, invasion, angiogenesis, and metastasis or apoptosis
[30]. For example, it has been shown that it is possible to attack genetic instability associated with cancer through nutritional strategies that inhibit proliferative signaling, attenuate oncogenic metabolism, and block inflammation
[31].
The biological activity of food polyphenols, a broad family of compounds with representatives in virtually all foods, has been specially studied for decades since they have in common an intense antioxidant activity that suggests other potential health outcomes, for example in breast cancer
[32]. Recently, intensive research has been carried out to determine the preventive or therapeutic activity of different natural phenolic compounds
[33], opening ways for its application in new treatments of various types of cancers such as breast
[34][35][36][37], colon
[38], or prostate
[39][40]. Some synthetic phenolic compounds have also been successfully studied for the treatment of some cancers
[41]. In addition to polyphenols, curcumin (diferuloylmethane) is one of the most studied foodstuffs in recent years as a potential therapeutic product for cancer and more specifically for leukemia
[42]. Traditional food products, such as rosemary extract, have been proposed as potential ingredients of precision nutritional supplements in cancer therapy, identifying molecular mechanisms related to the effects and the interactions with currently-used anticancer agents
[43]. In the case of colorectal cancer, lipid-metabolism-related genes have acquired relevant interest for precision nutrition therapies, since a wide range of tumorigenic steps can be influenced by lipid metabolism, both in primary tumors and distal metastasis
[44]. Certain therapeutic strategies based in diet patterns during adjuvant treatments are also sometimes considered as precision therapies
[45]. Finally, multi-targeting profiles of food ingredients are being investigated regarding their potential roles triggering anti-cancer molecular mechanisms through the modulation of certain gene expressions or signaling pathways
[46].
2. Current Insights on Precision Nutrition and Cancer Relapse Prevention
Since the same factors that are successfully driving precision medicine in cancer serve to design precision nutritional therapies, it is foreseeable that a new era in the treatment of cancer can be opened in coming years. Integration of nutritional strategies may be of special interest for patients treated with adjuvant therapy, in order to enhance therapy effects and to prevent cancer relapse. The knowledge regarding several bioactive foodstuffs mechanisms of action, and the fact that most of them address well established molecular targets, allows the transition into a potential clinical use. In the last decade, a growing number of research works have investigated the anticancer effects of bioactive natural products. However, only very recently have the molecular mechanisms by which nutrients may prevent relapse been explored.
Polyphenols have been the most studied family of compounds in decades for their different biological activities. In addition, some flavonoids have recently shown antitumor and antiproliferative activities that can be useful in relapse-preventive treatments. Epigallocatechin-gallate (EGCG), a flavonoid present in green tea, has been shown to inhibit tumor cell growth and increase apoptosis, promoting tumor suppression. This compound sensitizes human colon cancer cells to 5-fluorouracil, increasing the effects of adjuvant treatment and improving prognosis, and finally reducing tumor relapse risk
[47]. EGCG can also prevent lung cancer relapse in lung cancer mouse xenografts by blocking the cancer stem-cells-like growth through the modulation of the hsa-mir-485-5p/RXRα axis and downregulating protein acetylation in lung carcinoma cells
[48][49]. EGCG has been also been predicted to affect several pathways involved in cell death and survival, potentially leading to a reduced cancer progression
[50]. However, further molecular validations are needed in this sense. Another flavonoid with interesting features for relapse prevention is quercetin, which is present in vegetables such as onions. Quercetin promotes apoptosis and inhibits cell proliferation by modulating important signaling targets as PI3K/Akt or NF-κB effectively eliminating prostate CSCs. It also limits cell migratory capacity and progression of prostate cancer cell lines by the downregulation of MK
[51]. In breast cancer, quercetin can help preventing relapse by decreasing expression and activation levels of mTOR, PI3K and Akt proteins leading to a significant inhibition of MCF7 cancer cell proliferation
[52]. Another flavonoid with potentially interesting effects for the development of precision nutrition products is apigenin, present in fruits, vegetables, and food herbs such as parsley. This compound improves the effectiveness of adjuvant therapy with cisplatin enhancing both cytotoxicity and its anti-migratory effect on prostate cancer stem cells
[53]. Apigenin also helps preventing metastasis and relapse in non-small cell lung cancer cell lines and in an in vivo orthotopic bioluminescent xenograft model by inhibiting cell migration and invasion
[54]. Other interesting flavonoids are naringenin, obtained from citrus peel, which has been shown to inhibit proliferation and to induce apoptosis in prostate cancer cells
[55], and procyanidin-B2-3,3″-di-O-gallate (B2G2) extracted from grape seeds, that targets both differentiated cells and CSCs leading to tumor mass reduction
[56].
Curcumin is another bioactive food product that has been the subject of former numerous studies. Its anti-cancer effects, due its ability to modulate critical anti-apoptotic effectors such as Bcl-xl and NF-κB, are of prominent interest in potential relapse prevention treatments. Moreover, curcumin synergizes with vesicular stomatitis virus (VSV)-based oncolytic treatments modulating antiviral responses and components of the intrinsic apoptotic pathway in a prostate cancer cell model
[57]. Furthermore, in colorectal cancer, curcumin can modulate gene expression of HSPA5, SEC61B, G6PD, HMOX1, and PDE3B, affecting essential pathways like DNA replication or the cell cycle. On the other hand, the synergy of curcumin and oligomeric proanthocyanidins emerges as an opportunity to develop effective therapies, since both compounds share similar molecular mechanisms
[58]. Recently, relevant effects of curcumin in breast cancer cell models have been discovered, being that this compound is able to increase the expression of E-cadherin and decrease the expression of mesenchymal markers
[59]. It has been also shown that curcumin enhances the effect of some targeted drugs used in cancer such as gefitinib, an EGFR inhibitor, inducing autophagy-mediated apoptosis. This observation opens up opportunities for the use of this compound with treatments to prevent cancer relapse
[60].
Some lipid character food bioactives also show anti-cancer effects with mechanisms of action interesting for cancer relapse prevention. In this sense, docosahexaenoic acid (DHA) modulates the growth of colorectal cancer cells and induces expression of genes related to apoptosis
[61][62]. Also, β-sitosterol-d-glucoside has inhibitory effects on breast cancer cells growth
[63].
The studies mentioned above explain many of the effects on cell growth, tumor progression and metastasis of food bioactive products, opening promising opportunities for the application of these phytochemical foodstuffs in therapies for cancer relapse in breast, prostate, lung, and colon cancer. However, no studies of individual food products were found that applied to leukemia relapse. Regarding this type of cancer, only one paper was recovered, suggesting that additional research should be made in this sense. The cited article analyses the effects of a ginger extract rich in gingerols, demonstrating that this extract exerts a synergistic interaction with methotrexate with high antiproliferative impact in the drug-resistant leukemic sub-lines
[64]. Nevertheless, the mechanism that supports this effect is not explained. Indeed, the same happens with other food extracts recently studied and would require additional studies.
In general, interesting antitumoral, antiproliferative, or antimetastatic effects are demonstrated, however, the molecular mechanisms concerning these effects remain largely unknown in most cases. An exception may be the grape seed extract, rich in proanthocyanidins associated with antitumor effects in colorectal cancer in combination with curcumin, by regulating cell cycle and migration
[65]. Other extracts with identified effects on cell growth, tumor progression and metastasis in colorectal cancer are watercress extract, rich in phenethyl isothiocyanate
[66]; ginseng extract, rich in ginsenoside Rg3
[67]; and isodon extract, rich in flexicaulin A
[68]. Importantly, orange peel extract, rich in nobiletin, sinensetin, scutellarein tetramethylether, and tangeretin, exerts a synergistic interaction with 5-fluorouracil in colorectal cancer, modulating EMT transition, inhibiting cell proliferation and modulating cancer stemness, demonstrating a significant potential use of this combination in relapse preventive therapies
[69]. In any case, further research needs to be done in order to identify molecular mechanisms regarding the effects of these extracts. Although only individual compounds are now useful in precision nutritional strategies against well-identified therapeutic targets, these studies allow considering the extracts as an intermediate step towards the purification of the specific component responsible for the observed effect. In addition, the low toxicity of these extracts makes them suitable for other support therapies as nutritional supplements.
One of the barriers to be overcome in the application of bioactive phytochemical foodstuffs in cancer therapies is the frequent lack of bioavailability of these products. In this sense, new formulation strategies are being developed, such as bioactive carrier lipids in which the carrier not only increases bioavailability, but also provides a synergistic biological activity with the active ingredient. Interestingly, it has been reported that the use of shark liver oil rich in alkylglycerols as a bioactive lipid vehicle for rosemary extract shows synergistic effects in the expression of genes associated with immune modulation, inflammation, oxidative stress, lipid metabolism, and tumorigenesis in colorectal cancer
[70]. Besides this, a good example of a non-soluble and instable product is curcumin. This compound is emerging as a potent effector acting on numerous signaling and molecular pathways that regulate tumor growth and cancer relapse. Despite the growing knowledge about its properties, curcumin cannot be approved as a therapeutic compound due to its limitations in terms of bioavailability and stability. Regarding this, several formulation studies are being performed and a significant number of cases have provided better therapeutic results than the individual bioactive
[71][72][73][74][75].
3. Conclusions
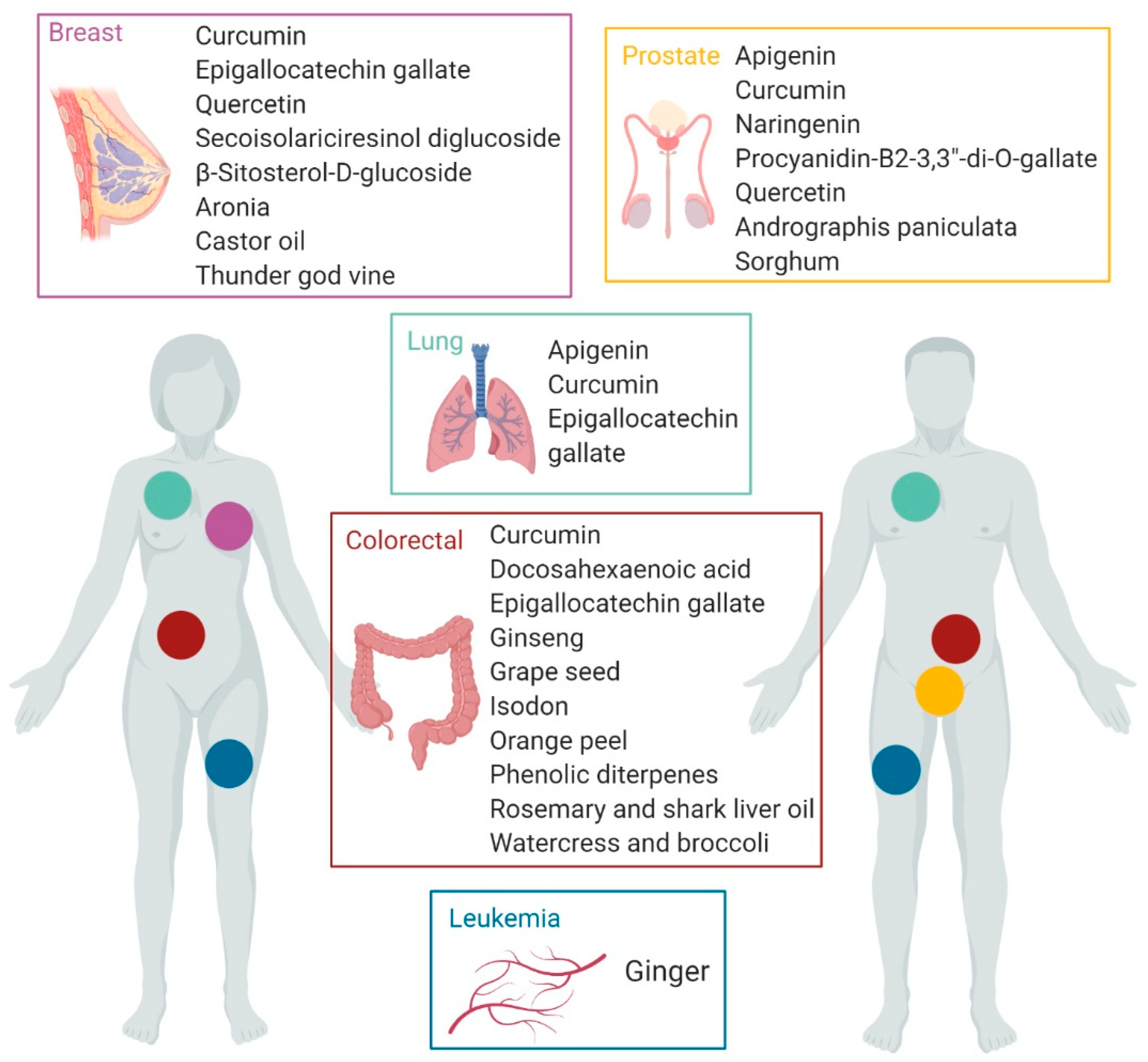
Although, in the context of cancer research, studies that refer to nutritional therapies based on the use of bioactive foodstuffs in adjuvant treatments are still limited, current results are encouraging since there are several phytochemical bioactive foodstuffs with proven modulating effects of tumor growth, progression, and metastasis, and therefore can be tested in humans with a reasonable probability of success if they are applied in cancer relapse treatments (Figure 1). In this sense, the compounds currently identified for this purpose are in general extensively-studied products in the last years such as the polyphenols apigenin, epigallocatechin gallate, procyanidin B2-3,3-di-O-gallate, quercetin, naringenin, secoisolariciresinol diglucoside, and curcumin, as well as docosahexaenoic acid and β-Sitosterol-d-glucoside lipids. Among the targeted cancers included in this work, lung and colon cancer are being widely studied, and prostate and breast cancer have concentrated the largest number of applications. However, there is a great lack of studies related to the use of phytochemical foodstuffs in leukemia therapies and more studies are needed in this disease. The possibilities of using foodstuffs in cancer treatments and more specifically anti-relapse therapies, are being reinforced with the development of formulation technologies that significantly increase the efficiency of these products.
Figure 1. Bioactive foodstuffs and natural extracts with proven effects in cancer treatment. (created with BioRender).
Cancer heterogeneity is one of the features of this disease that makes its treatment particularly challenging. Innovative therapies for patients are continuously being tested and progress has been made in the last years developing early diagnosis protocols and improving patient prognosis. However, despite these efforts, a high percentage of patients relapse after surgery or initial therapy. Cancer relapse involves a great number of different molecular mechanisms that vary form one patient to another. This points to precision medicine as a key element to personalize cancer treatment and prevent relapse, and suggest the value of new effective and safe compounds that potentiate the effects of already-known chemotherapy agents. In this sense, the growing number of studies regarding the mechanisms of several bioactive foodstuffs in the treatment of different types of cancer, open a new layer in precision cancer therapy. Its association with specific genetic targets or different molecular pathways inhibiting tumor growth and metastasis constitutes an important customization component. Moreover, it shows high synergism with several chemotherapy drugs, acting as enhancers of these anti-tumor effects or even sensitizing and reverting chemotherapy resistance. In essence, these products are emerging as novel complementary agents that can be useful in precision nutrition therapies addressing relapse prevention in treatments of cancerous processes.