1000/1000
Hot
Most Recent

Electroencephalography (EEG) is a noninvasive, safe, and relatively convenient technique to record brain activities, which allows quantitative methods to detect changes and patterns of EEG signals related to DOC. The best feature of EEG data is neural oscillations. From the perspective of biophysics, EEGs are extracellular currents that reflect the total dendritic postsynaptic potentials in millions of parallel pyramidal cells.
Due to advances in critical care, an increasing number of patients survive acute brain injury, causing an increased incidence and prevalence of patients with disorders of consciousness (DOC). DOC encompasses coma, vegetative state (VS)/unresponsive wakefulness syndrome (UWS), and minimally conscious state (MCS). Patients with UWS have a sleep–wake cycle, but they completely lose their awareness of themselves and their surroundings [1], while patients with MCS have awareness and show purposeful behaviors but are unable to communicate effectively [2]. Recently, MCS has been further divided into two substates, MCS+ (high-level behavioral responses, such as command following) and MCS− (low-level behavioral responses, such as visual pursuit and pain localization) [3]. The gold standard Coma Recovery Scale-Revised (CRS-R) is the best behavioral assessment criterion for the diagnosis of UWS or MCS, but patients who are unable to follow the commands due to motor impairments may receive an incorrect diagnosis of UWS. Therefore, the misdiagnosis rate has been reported to be as high as 43% [4].
From a neurobiological viewpoint, consciousness and sleep are intimately linked [5]. Regular sleep patterns could reflect the preservation of brain functions [6] and play a key role in memory consolidation [7], hormonal regulation [8], and immune functions [9]. A better understanding of sleep-in patients with DOC could be of great help in distinguishing patients with different levels of consciousness. Subsequent findings further demonstrated that the integrity of identified sleep patterns carries important prognostic information for outcomes of consciousness recovery [10]. Consequently, the diagnostic and prognostic value of sleep in DOC has received increasing attention.
Neurophysiological changes in sleep have been well studied in healthy humans [11]. However, more detailed sleep assessment for DOC patients is still a controversial issue. Some research groups believe that manual sleep staging is feasible [12][13], while others [14] believe that it is impossible to perform sleep staging according to the established criteria of the American Academy of Sleep Medicine (AASM) or Rechtschaffen and Kales (R&K). Furthermore, the frequency, topography, power, and shape of EEG or PSG signals are changed in patients with severe brain injury. It is difficult to find polysomnographic patterns, such as sleep spindles, K-complex, and rapid eye movement.
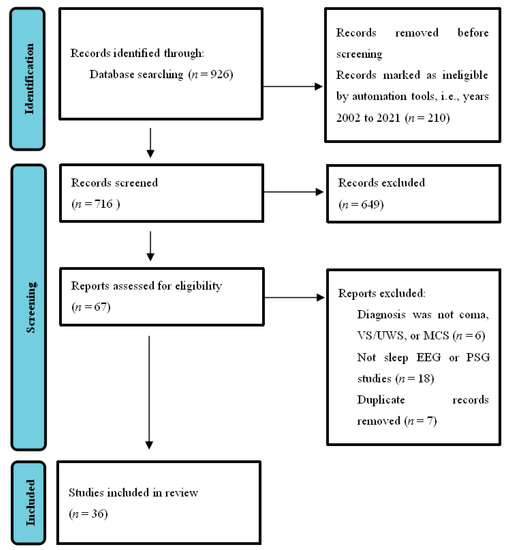
This entry was conducted according to PRISMA guidelines [15]. As shown in Figure 1 ,researchers searched the PubMed database using the concepts of sleep and DOC. Search words included ((disorders of consciousness) OR (DOC)) AND (sleep)), and the field search was (title/abstract). The number of journal papers found from 2002–2021 was 716. We emphasized the articles published in the last 20 years regarding the diagnosis and classification of DOC without language restrictions. Exclusion ( n = 649) were records not closely related to the classification, diagnosis, and prognosis of DOC, which mainly included the studies of the state of consciousness under sleep or anesthesia, the studies of sleep under different lifestyles, the studies of the clinical manifestations of patients with schizophrenia, Alzheimer’s disease, or other mental disorders using different drugs, and the studies involving other types of measurements, such as functional magnetic resonance imaging (fMRI), positron emission tomography (PET) or transcranial magnetic stimulation (TMS), or duplicates. The final result was that 36 articles were included. We focused on articles using sleep EEG or PSG methods, as well as studies on patients diagnosed with coma, VS/UWS, or MCS. We believe that it is a good time to summarize new technologies and address the gap between theory and application in this field.
Studies related to the topic of sleep EEG in patients with DOC can be devised into three main classes. First, each sleep state is characterized by a different type of EEG activity, and thus EEG analysis has been used for sleep stage classification. Second, using typical sleep EEG waveforms for detection and diagnosis in patients with DOC has been reported by some researchers [12][15][16]. Third, sleep EEG changes have a predictive value in patients with DOC. The state-of-the-art of the above three classes of sleep EEG are reviewed in the following sections.
PSG is the main tool to evaluate sleep in the laboratory and can be used for clinical and research purposes. PSG is used to collect EEG, EOG, EMG, electrocardiogram, pulse oximetry, airflow, and respiratory effort during sleep and utilize these to evaluate the underlying causes of sleep disturbances [17]. PSG can provide much information about the integrity of the global brain network; thus, sleep assessment can contribute to the diagnosis of DOC patients. During PSG monitoring, EEG and other sensors are used to divide sleep into several distinct stages. Sleep can be roughly divided into NREM sleep and REM sleep. The sleep stages cycles from NREM sleep stage 1 (N1) to REM sleep and then starts again from stage N1. A complete sleep cycle takes approximately 90 to 110 min, and each stage lasts 5 to 15 min. Figure 2 describes the distinct sleep stages of healthy subjects for more than 8 h. The sleep staging process can be very complicated. Many parameters of sleep staging need to be taken into consideration at the same time, and the contextual epoch scores also need to be considered. Compared with records from healthy subjects, scoring records from subjects with specific sleep disorders can be more challenging.
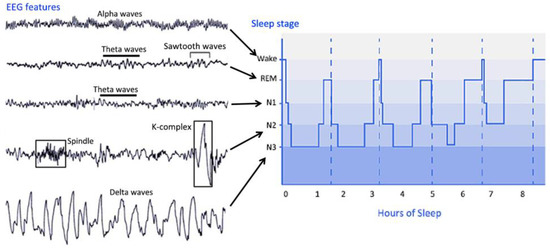
In 2007, the new AASM Manual for the Scoring of Sleep and Associated Events was published, which provides a comprehensive reference for the assessment of PSG data. According to the new standard, the following staging system was proposed. AASM sleep staging is divided into five stages, including stage W (wakefulness), stage R (REM sleep), N1 stage (non-REM sleep stage 1), N2 stage (non-REM sleep stage 2), and N3 (non-REM sleep stage 3). Unlike the sleep staging of the R&K standard published in 1968, the new standard has incorporated stage 3 sleep (S3) and stage 4 sleep (S4) into N3. The characteristics of the sleep stages are summarized as follows.
The characteristic of stage W is the appearance of an alpha rhythm in the EEG signal. Theta waves can be observed in stage N1. Sleep spindles and K-complexes may be detected in stage N2. Stage N3 is the deep sleep stage of sleep, in which slow waves and delta waves are dominant in the EEG signal. In addition, spindles may occur at this stage. In stage REM, rapid eye movements occur, and there is no obvious characteristic in the EEG signal. Theta waves and possible sawtooth waves in the EEG signal can be observed [18].
Sleep parameters such as sleep spindle, slow wave sleep, and rapid eye movement sleep can be used as independent markers of the severity of consciousness impairment. The purpose of sleep staging is to identify the stages of sleep that are crucial in the diagnosis and treatment of sleep disorders. The principle of sleep staging is to divide the night into continuous periods of 30 s, called epochs. Traditionally, doctors use these epochs to assess and analyze sleep patterns. However, the traditional manual sleep staging method is time-consuming and relies on the experience of doctors. Therefore, automatic sleep staging methods have become very important in recent years.
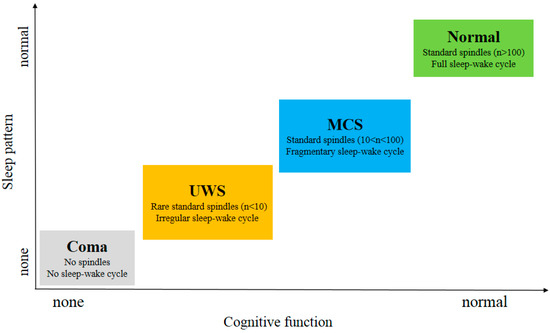
As shown in Figure 3 , subjects in MCS and Normal showed all stages of sleep in contrast to UWS patients. This indicates that the brain function of these patients has been fully protected [19]. MCS patients exhibit relatively preserved thalamocortical connectivity compared to UWS patients. Compared to MCS patients, the UWS patients did not show homoeostatic regulation, a detectable sleep cycle or slow wave activity. Since slow wave activity is considered to be related to plasticity [20], the better prognosis observed in the studies comparing patients with MCS to those with UWS can also be partially explained in their findings [21]. S. De Salvo et al. [22] proposed a system named Neurowave to monitor event-related potentials (ERPs) evoked by neurosensory stimulation in 11 VS and 5 MCS patients. The absence of an ERP component could be a distinctive marker between VS and MCS patients. The other differences in sleep elements between UWS and MCS patients are summarized in Table 1 .
| Reference | N (UWS/MCS) | Sleep–Wake Cycle | SWS | REM | Spindles | Main Results |
|---|---|---|---|---|---|---|
| Landsness et al. (2011) [2] | 5/6 | 5/5 UWS 6/6 MCS |
not reported | 0/5 UWS 5/6 MCS |
0/5 UWS 6/6 MCS |
MCS showed an alternating sleep pattern; UWS preserved behavioral sleep but no sleep EEG patterns; |
| Cologan et al. (2013) [1] | 10/10 | 3/10 UWS 5/10 MCS |
4/10 UWS 7/10 MCS |
3/10 UWS 9/10 MCS |
4/10 UWS 6/10 MCS |
The presence of rest periods did not always indicate retention electrophysiological sleep–wake cycles that should no longer be used to differentiate UWS from MCS |
| Forgacs et al. (2014) [23] | 8/23 | 5/8 UWS 22/23 MCS |
2/8 UWS 13/23 MCS |
2/8 UWS 9/23 MCS |
4/8 UWS 18/23 MCS |
EEG was well organized in patients with evidence of concealed command-following; Preservation of specific EEG characteristic could be used to differentiate UWS from MCS; |
| De Biase et al. (2014) [5] | 27/5 | 22/27 UWS 5/5 MCS |
not reported | 4/27 UWS 5/5 MCS |
15/27 UWS 5/5 MCS |
The concomitant presence of sleep spindles and REM sleep correlated with patients diagnosis |
| Aricò et al. (2015) [24] | 8/6 | 5/8 UWS 6/6 MCS |
not reported | 2/8 UWS 5/6 MCS |
1/8 UWS 4/6 MCS |
MCS showed more preserved sleep pattern, preserved NREM/REM sleep distribution, and physiologic hypnic figures than UWS |
| Arnaldi et al. (2016) [10] | 20/6 | 17/20 UWS 6/6 MCS |
not reported | 5/20 UWS 3/6 MCS |
17/20 UWS 6/6 MCS |
The boundaries between UWS and MCS were elusive |
| Sebastiano et al. (2018) [25] | 55/31 | not reported | 16/55 UWS 31/31 MCS |
23/55 UWS 21/31 MCS |
5/55 UWS 8/31 MCS |
The presence of SWS was the most appropriate factor to differentiate UWS from MCS |
| Gibson et al. (2020) [26] | 8/3 | 8/8 UWS 3/3 MCS |
4/8 UWS 3/3 MCS |
5/8 UWS 3/3 MCS |
4/8 UWS 1/3 MCS |
MCS tended to exhibit more preserved sleep pattern than UWS |
The emergence of the eye-opening periods, the reappearance of the circadian rhythm and the behavioral sleep–wake cycles prove that DOC patients come from a coma to MCS [27]. Recently, Blume et al. [28] reported that the integrity of patients’ circadian rhythms was associated with arousal levels, possibly due to better depiction of periods of sleep and wakefulness. By observing the behavior of patients with DOC for prolonged eye-opening or eye-closing periods, it can be inferred whether there is a circadian rhythm sleep–wake cycle in DOC patients. However, although the existence of a sleep–wake cycle is important for differential diagnosis, there is little evidence that patients with DOC exhibit circadian rhythms or sleep–wake cycles similar to those of healthy people. Past studies [29][30] have reported differences in the circadian activity of most patients with UWS and MCS, and the signs of circadian rhythm in MCS patients are more pronounced.
Oksenberg et al. [31] found that the phasic activities of rapid eye movement sleep in 11 UWS patients were significantly reduced, but the number of these activities had nothing to do with the recovery of the clinical condition because experiments indicated that there was no obvious difference in rapid eye movement sleep phasic activities between the patients who recovered consciousness and those who did not. UWS is caused by overwhelming damage to the cerebral hemisphere, resulting in a large loss of cortical activity but retaining the functional brain stem, allowing continuous regulation of primitive reflexes and vegetative functions. Although UWS retains the brain stem mechanism responsible for the sleep wake cycle and the emergence of rapid eye movement sleep, the significant decrease in rapid eye movement sleep phase activity indicates that other brain stem mechanisms are impaired [31].
Sleep spindles are relatively lacking in patients with DOC, and the abnormalities of sleep spindles in patients with UWS are greater than those in MCS patients. A few UWS patients and most MCS patients had preserved spindles, SWS, and rapid eye movement sleep [1]. In one study [23], Forgacs et al. described the characteristics of 44 DOC patients with conventional EEG. In approximately one-third of UWS patients and in more than half of MCS patients, preserved sleep spindles, rapid eye movement, and slow-wave sleep can be seen. Moreover, the presence, quality and quantity of sleep spindles in both patients with UWS and MCS were associated with more favorable outcomes. In addition, based on their research findings, UWS or MCS patients who have severely abnormal EEG background activity will be less likely to have a high level of cognitive functions demonstrated by functional neuroimaging.