1000/1000
Hot
Most Recent

Studies have shown that there is a disparity between males and females in south-east Asia with regard to oral cancer morbidity. XIST may play an important role in oral cancer morbidity when associated with sex. Lack of salivary lncRNA XIST expression was associated with an increased risk of oral squamous cell carcinoma (OSCC).
According to global statistics published by the World Health Organization, oral cavity cancer is among the most prevalent types of cancer worldwide, with the female to male incidence ratio showing a discrepancy of 2:1 in south-east Asia [1]. Excessive alcohol consumption, betel quid chewing, and cigarette smoking (ABC habits) are risk factors for oral cancer [2]. However, the ABC habits cannot explain the increasing trend of young females diagnosed with oral squamous cell carcinoma (OSCC) without performing the ABC habits[3].
The long non-coding RNA XIST is an X-linked gene that contributes to X-chromosome inactivation. It is also related to tumorigenesis and progression in nasopharyngeal carcinoma , small intestinal adenocarcinoma , and breast cancer [4][5][6]. A previous study revealed that a loss of genomic copy number variants of XIST is shown in the OSCC group[7]. Recently, one research article provided evidence of a relationship between XIST and the inhibition of tumor progression in vitro [8].
Among the 102 participants, 59 were patients with OSCC (male n = 33, female n = 26) and 43 were individuals without OSCC (the control group) (male n = 16, female n = 27). The average ages of male and female patients were 53.9 (2.2) and 58.2 (2.3) years old, respectively. The average ages of male and female individuals in the control group were 49.7(2.5) and 39.1(1.3) years old, respectively. Salivary lncRNA XIST was only expressed in females. Among the OSCC group, 35.6% consumed alcohol, 40.7% had a betel nut chewing habit, and 52.5% smoked cigarettes. For primary tumors, 47.5% of cases were T1-T2, and 52.5% were T3-T4. Additionally, 50.8%, 40.7%, and 8.5% of tumors were well, moderately, and poorly differentiated, respectively. For clinical stages, 35.6% of cases were I-II, and 64.4% were III-IV. Only two patients (3.4%) had distant metastasis. No patients showed tumor recurrence. The tumor sites involved were 28.8% buccal, 33.9% tongue, and 37.3% others, including gingiva, floor of the mouth, mandible, and palate (Table 1).
| OSCC n = 59 | Control n = 43 | |
|---|---|---|
| Average age, y (mean ± SD) | ||
| Male | 53.9 ± 2.2 | 49.7 ± 2.5 |
| Female | 58.2 ± 2.3 | 39.1 ± 1.3 |
| Variable | n (%) | n (%) |
| Sex | ||
| Male | 33 (55.9) | 16 (37.2) |
| Female | 26 (44.1) | 27 (62.8) |
| Salivary lncRNA XIST expression | ||
| Male | 0 | 0 |
| Female | 3 (11%) | 22 (81%) |
| Alcohol drinking | ||
| Yes | 21 (35.6) | 0 (0) |
| No | 38 (64.4) | 43 (100) |
| Betel nut chewing | ||
| Yes | 24 (40.7) | 0 (0) |
| No | 35(59.3) | 43 (100) |
| Cigarette smoking | ||
| Yes | 31 (52.5) | 0 (0) |
| No | 28(47.5) | 43 (100) |
| Primary tumor stage | ||
| T1-T2 | 28 (47.5) | |
| T3-T4 | 31 (52.5) | |
| Differentiation | ||
| Well | 30 (50.8) | |
| Moderate | 24 (40.7) | |
| Poor | 5 (8.5) | |
| Clinical stage | ||
| I-II | 21 (35.6) | |
| III-IV | 38 (64.4) | |
| Distant metastasis (M) | ||
| Yes | 2 (3.4) | |
| No | 57 (96.6) | |
| Recurrence | ||
| Yes | 0 (0) | |
| No | 59 (100) | |
| Tumor site | ||
| Buccal | 17 (28.8) | |
| Tongue | 20 (33.9) | |
| Others | 22 (37.3) |
A preliminary test to detect XIST expression in buccal cells and saliva, samples of which were kindly provided by four healthy research assistants (two males and two females) was conducted. Of the volunteers, two males and one female did not express XIST in the buccal cells or in the saliva (data shown in Supplementary Figure S1). Salivary lncRNA XIST was only expressed in females, with a high proportion observed in control group females (Table 1, Figure 1). Control group and OSCC males lacked salivary XIST expression with detectable GAPDH amplicons (data shown in Supplementary Figure S2).
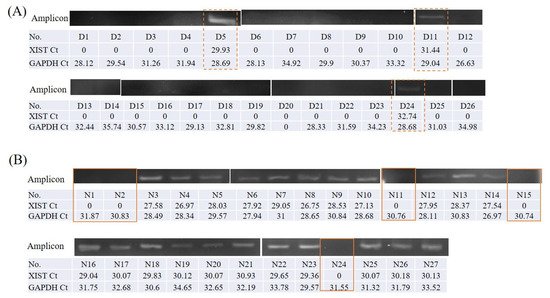
The grouping gels, which were cropped from different part of the same gel, or from different gels, were shown with a space. The original full-length gels were included in the supplementary files during peer review process.
Among the patients with OSCC, 83% (20 of 24) of the smokers, 90.3% (28 of 31) of those who consumed alcohol, and 95% (20 of 21) of those chewed betel nuts were male. Tumors of male patients were low-grade or well differentiated in 66% (22 of 33) of cases, and most were in the buccal site (13 of 33). A higher proportion of tumors in female patients showed moderate or poor differentiation (17 of 26), and most were on the tongue (14 of 26) (Table 2). Most females with OSCC did not have ABC habits. The tumor was typically small and poorly differentiated when located in the tongue. Most males with OSCC had ABC habits, and the tumors were typically located in the buccal site, were larger, and well differentiated.
| Sex | |||
|---|---|---|---|
| Variable | Male n = 33 |
Female n = 26 |
p |
| Smoking | |||
| Yes | 20 | 4 | <0.001 *** |
| No | 13 | 22 | |
| Alcohol drinking | |||
| Yes | 28 | 3 | <0.001 *** |
| No | 5 | 23 | |
| Betel nut chewing | |||
| Yes | 20 | 1 | <0.001 *** |
| No | 13 | 25 | |
| Differentiation | |||
| Low grade or well | 22 | 9 | 0.019 * |
| moderate or poor | 11 | 17 | |
| Diagnosis | |||
| Tongue Ca. | 6 | 14 | 0.026 * |
| Buccal Ca. | 13 | 4 | |
| Gingiva Ca. | 7 | 5 | |
| Others | 7 | 3 | |
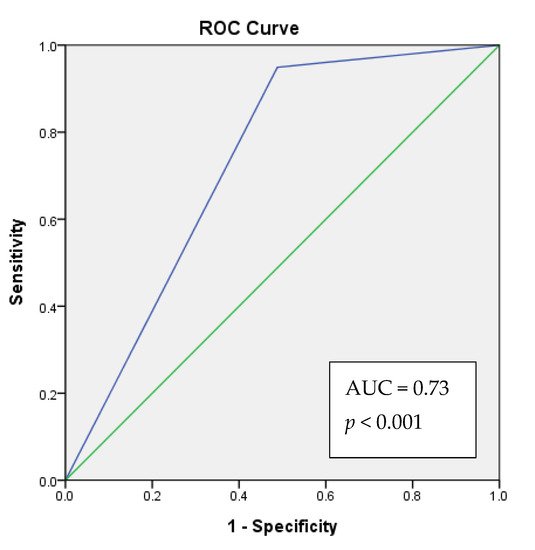
Study analyzed the correlation between the clinical–pathological data and XIST expression. Salivary lncRNA XIST expression was correlated with sex (Table 1 and Table 3) among all participants, and was correlated with OSCC among female participants (Table 4). Salivary lncRNA XIST expression had no significant correlation with ABC habits or death. We further conducted binomial logistic regression, and found that individuals who did not express XIST had a 19.5-fold higher risk of suffering from OSCC. Females who did not express salivary lncRNA XIST had a 33.7-fold higher risk of suffering from OSCC (Table 5). The ROC analysis showed that, 73% (acceptable discrimination) of the time, the model would correctly assign a higher absolute OSCC risk to patient with an absence of XIST expression (Figure 2).
| Sex | Alcohol | Betel | Cigarette | Death | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| XIST expression | F | M | No | Yes | No | Yes | No | Yes | No | Yes |
| Yes | 3 | 0 | 3 | 0 | 3 | 0 | 2 | 1 | 3 | 0 |
| No | 23 | 33 | 25 | 31 | 35 | 21 | 33 | 23 | 38 | 18 |
| Fishe’s exact test p (two-tailed) |
0.08 | 0.1 | 0.546 | 1 | 0.546 | |||||
| Phi | 0.261 * | 0.244 | 0.172 | 0.035 | 0.153 | |||||
| OSCC | Alcohol | Betel Nut | Cigarette | |||||
|---|---|---|---|---|---|---|---|---|
| XIST expression | No | Yes | No | Yes | No | Yes | No | Yes |
| Yes | 22 | 3 | 25 | 0 | 25 | 0 | 24 | 1 |
| No | 5 | 23 | 25 | 3 | 27 | 1 | 25 | 3 |
| Fisher exact test p (two-tail) |
<0.001 | 0.238 | 1 | 0.613 | ||||
| Phi | 0.7 *** | 0.231 | 0.131 | 0.127 | ||||
| B | S.E. | p | OR | ||
|---|---|---|---|---|---|
| All Participants n = 102 |
XIST expression | 2.973 | 0.667 | <0.001 | 19.556 |
| constant | −1.992 | 0.615 | 0.001 | 0.136 | |
| Female subjects n = 53 |
XIST expression | 3.518 | 0.789 | <0.001 | 33.733 |
| constant | −1.992 | 0.615 | 0.001 | 0.136 | |
A patient who lacks salivary XIST expression will have a higher predicted OSCC risk score than a patient with salivary XIST expression. The model will correctly assign a higher absolute OSCC risk to a patient with an absence of XIST expression 73% (acceptable discrimination) of the time.