1000/1000
Hot
Most Recent

Free radicals are atoms, molecules or ions with one or more unpaired electrons. The most reactive ones have high reduction potentials; i.e., they readily oxidize most molecules indiscriminately. There is a common misconception that all free radicals are highly reactive, but in fact the range of those able to cause biological damage is quite narrow: it is made up principally of hydroxyl, peroxyl, alkoxyl, thiyl, phenoxyl and semiquinone free radicals, and high valence transition ions.
Oxidative stress is commonly coupled with excessive formation of free radicals, with some researchers actually identifying free radicals as the origin and cause of OS. There is a common misconception that all free radicals are highly reactive, but in fact the range of those able to cause biological damage is quite narrow: it is made up principally of hydroxyl, peroxyl, alkoxyl, thiyl, phenoxyl and semiquinone free radicals, and high valence transition ions. The usual form of damage is abstraction of an electron or H atom from a target molecule, creating a new, less reactive secondary target free radical. In complex biological systems, the result is a chain of successive electron transfers, creating new free radicals with decreasing reactivity, as required by thermodynamics. If the chain involves critical molecules, such as DNA, proteins or lipids repaired or replaced only slowly, the result may be a permanent impairment of a vital function, which, if not reversed, can constitute the first step in the development of a disease or another form of damage. The radical chain is only terminated by reaction with another free radical, a transition metal ion or with an antioxidant, with the last creating a radical no longer able to propagate the damage.
In discussing the phenomenon of oxidative reactions of free radicals and their consequences, it has to be noted that low levels of free radicals form continuously in vivo and fulfil significant roles in tissue defence, DNA biosynthesis, redox regulation and possibly in thiol-based cell signalling [1][2][3][4][5][6]. There is therefore a clear and important distinction between the consequences of formation of low and excessive levels of free radicals; the former are essential and not a danger to the organism because its antioxidant defences can prevent or repair any collateral molecular damage, while the latter can trigger or aggravate a wide range of diseases and other undesirable conditions.
The initial reaction of the primary damaging free radicals with biomolecules commonly produces carbon-centred (C-centred) free radicals. Their principal subsequent reactions result in new free radical chains, which include formation of peroxyl free radicals, ROO● , in a fast reaction with physiological oxygen ( Scheme 1 ).
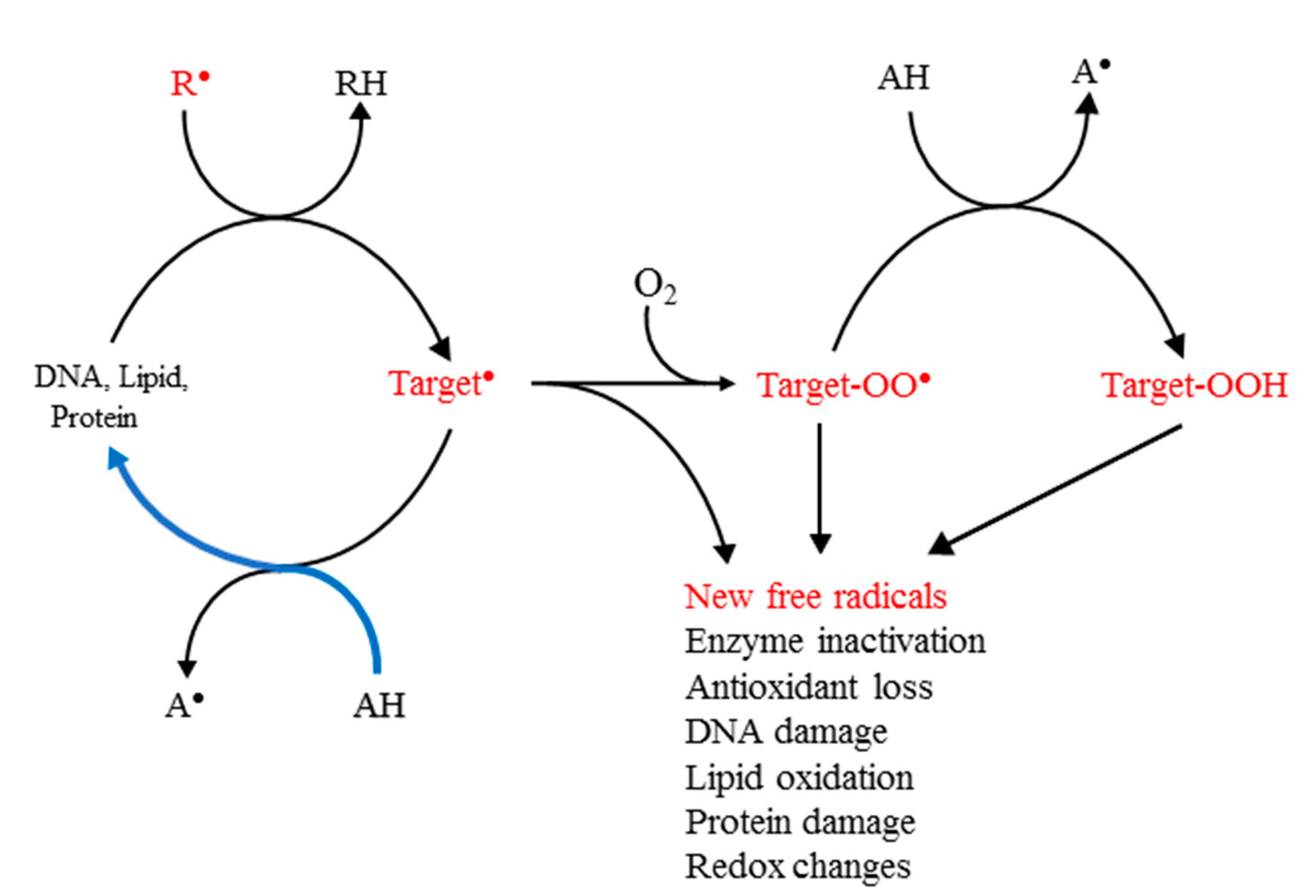
Scheme 1. Principal biological targets of the primary free radicals and damage repair. R● is the initiating free radical and AH an antioxidant. The initial reaction produces a carbon-centred free radical in the target. Reactive species are shown in red. The blue curve shows the flow of the reducing equivalents under optimal prevention of damage.
In the reducing environment of cells and tissues, peroxyl radicals are commonly converted to hydroperoxides. All these species, namely, the C-centred and other free radicals, peroxyl radicals and hydroperoxides, have the capacity to propagate the damage triggered by R● , with peroxyl radicals believed to be the main carriers of damage in living organisms; many of the commonly used assays for the formation of free radicals in cells and tissues depend on the detection of peroxyl radicals and hydroperoxides [7][8].
Defence against free radical-induced damage can be achieved by scavenging the R● or repair of the damaged target molecules. Under oxidative stress, this should be achievable in theory by enhancing the levels of the endogenous antioxidants or, if necessary, supplementation with additional antioxidants. Considerations of the mechanism of action of the most damaging free radicals, such as the hydroxyl, HO● , have shown that, because of their high reactivity and low levels in vivo, direct scavenging by added radical scavengers is not feasible, despite a commonly held contrary view [9]. This means that the earliest and most effective point for protective action is the secondary target free radical, because its repair prevents subsequent molecular damage ( Scheme 1 ). It is important to note that the well-documented recognition of the signalling and defensive functions of ROS in vivo means that the role of antioxidants in living organisms is not to eliminate all free radicals, but rather to lower any excessive levels, so that they can be neutralised by the endogenous antioxidants present.
The practical possibility of modification of biological damage by antioxidants is based on extensive chemical knowledge of the properties of free radicals, which established that their effectiveness is largely determined by thermodynamics and kinetics [10][11]. The former is an intrinsic property of the reacting species and cannot be altered. Importantly, however, kinetic factors can be manipulated because they depend on concentrations of the reactants: