The almond (
Prunus dulcis Mill.) is a member of the
Rosaceae family and is considered a native plant from Minor Asia
[12], being one of the oldest nut trees cultivated worldwide with special relevance in the Mediterranean warm-arid countries
[13][14], namely the Apulia region on southern Italy
[15]. Among tree nuts, almonds present as one of the most important nuts, which is very noticeable in tree nut production data around the world (Walnut 3663, Almond 3183 and Hazelnut 864 ktons/year;
[16]). Furthermore, its nutritional properties should be highlighted; high levels of mono and polyunsaturated fatty acids, phytosterols and a low glycemic index are associated with reduction of some risk factors for cardiovascular disease and diabetes
[17][18][19][20]. It has also been described as having antioxidant and inflammatory activities due to its polyphenol content, including flavonoids, hepato and neuroprotective potential and, perhaps the most known, cholesterol-lowering properties
[21][22][23][24]. Also, almond derived products such as their oils have demonstrated both antibacterial and antifungal capabilities
[25] which makes almond a product of great interest both to the consumer and producer.
Regarding almond cultivars, European commercial cultivars such as the Spanish Marcona, Glorieta, Masbovera, Guara and Francolí cvs. and the French Ferrastar, Ferraduel and Ferragnès cvs. are the main ones produced in Europe. In the United States, the most widely produced almond variety in Nonpareil cv. represents near half of the production. On other hand, in Portugal there is a mix of traditional and local varieties such as Amendoão, Pegarinhos, Casanova and Refego cvs.
[26][27]. However, in a study testing three almond varieties Nonpareil, Mission and Carmel against eight almond allergic patient’s sera, no significant differences were found. New similar research must be conducted to correctly evaluate the allergic potential of each variety of interest
[28].
Along with the almond nutritional value comes the agronomical properties of different cultivars. For example, Bolling et al.
[29] described that the individual polyphenols synthesis was only due to the cultivar itself, however total polyphenols and antioxidant activity were significantly dependent on both genotype and environmental growing conditions. Pursuing this point of view, Summo et al.
[30] performed a study aiming to determine if either the cultivar or harvest time influence the chemical composition of the fruit. From this, the team concluded that, in fact, harvest time and genotype both have a strong influence on the fruit nutritional value.
2.1. Almond Allergy
Nut allergy is associated with clinical symptoms that can range in severity from mild to life-threatening, and in this sense when a patient is diagnosed with an allergy to a certain nut it is often advised to avoid the consumption of the entire group
[31][32].
Epidemiologically speaking, almond allergies have the fourth highest prevalence among the tree nuts allergies
[33]. Looking at the specific cases of the United States, Korea, United Kingdom, Mexico and Sweden, almonds present the third most common tree nut to cause allergies in the United States
[10], and between 9% and 15% of people pre-sensitized to tree nuts also report allergy to almonds
[34]. In a study performed in a group of 134 Korean patients with previous reports of food allergies, 11.2% also reported almond allergies. Among them, 16.3% were between 19 to 29 years old, 13% in the 40–49 age group and 9.1% in the 50–59 group. Also, the same study reported that sensitivity to almonds is lower in females, with 9.8% compared to males at 13.5%
[35]. In the United Kingdom, in pre-sensitized individuals, almonds represent the most common tree nut allergy, with 22% to 33% of the cases
[34][36]. The higher rate of sensitization to almonds was reported in a study performed in Mexico City, reporting a 43% rate in older children with ages comprised between 6 and 17 years old
[37]. A cross-sectional enquiry made in Sweden with 1042 responses from individuals between 17 and 78 years old concluded that near 32.5% of adults had food hypersensitivity and 3% were sensitive to almonds
[38].
Almond allergy can cause several clinical responses. The Oral Allergy Syndrome (OAS) is a pollen-food syndrome that produces mild oral symptoms in cases of pollen sensitization triggered by nuts. Although it hardly causes anaphylaxis, it can happen in the direct confrontation of serum sIgE with PR-10 homologous
[39]. Another common clinical response is allergic rhinitis, that has been associated with almond allergies in a study performed in southern Taiwan with a group of 216 individuals with ages comprised between 2 and 93 years old. Most of these people had respiratory and cutaneous symptoms, and the study reported a 36.97% prevalence of allergic rhinitis caused by almonds in the group of the non-sensitized patients. Besides allergic rhinitis, asthma has been associated with almonds with a prevalence of 7.4% in the non-sensitized nut group and 13.70% in the sensitized one. Also in Taiwan, it was reported that almonds were responsible for 42.47% of atopic dermatitis cases in a group of 33 nut sensitized individuals
[40]. Other symptoms can emerge, such as gastrointestinal ones. In a group of 1024 sensitive individuals, 15% reported these, and from those, 2.7% were due to almonds
[38].
Regarding strategies for prevention and therapy for an almond allergy, the main method is dietary avoidance. Individuals sensitive to almonds should take special attention looking at packages and labels to prevent the ingestion of almond or almond-based products
[39]. However, there are some strategies that seem to prevent the development of almond allergies, namely the premature consumption of almonds during infancy or even during pregnancy, or lactation also showed a positive impact on its prevention
[41]. Moreover, there is evidence that about 10% of tree nut allergies are outgrown by young individuals who develop tolerance due to the rise of T regulatory cells and the consequent reduction of allergen specific IgE
[42]. Immunotherapy, a food allergen-specific therapy, which refers to the administration of gradual and increasing doses of an antigen over a certain time
[43][44], is considered as a solid option since in the majority of cases the side effects are mild, such as itching and, if successful, immunotherapy can induce desensitization and less commonly sustained unresponsiveness, also known as tolerance
[5]. Moore, Stewart and Deshazo
[5] believe that tolerance induced by immunotherapy with or without the administration of monoclonal antibodies could significantly shift the allergic diseases field.
Cross reactivity between almonds and other sources of allergens is a well-known problem and there are some of these associations (summarily described in Table 1) already described.
Table 1. Almonds’ most common cross reactions with other relevant sources of allergens. Green areas represent a positive association between almond allergens and other allergens of the respective sources.
| |
Possible Cross-Reaction Source |
| Source |
Allergen |
Mahleb |
Peanut |
Chestnut |
Hazelnut |
Walnut |
Peach |
Pollen |
Profilin-Containing Plants |
Maze |
| Almond |
Pru du 3 |
|
|
[45] |
|
|
|
| Pru du 6 |
[46] |
|
|
|
|
|
|
|
[47] |
| Pru du 1 |
|
|
|
|
|
|
[39] |
|
|
| Pru du 4 |
|
|
|
|
|
|
|
[48] |
|
| Pru du γ-conglutin |
|
[49] |
|
|
|
|
|
|
|
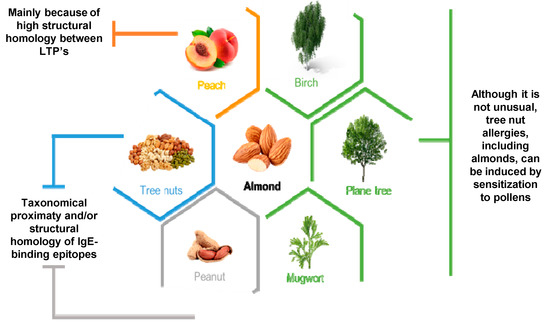
Nevertheless, it is still unclear if the taxonomic proximity between tree nuts groups and peanuts is a key factor for the cross-reactivity between these two, or it comes from the high structural homology of IgE-binding epitopes
[50][51]. In general, tree nut allergies are caused by non-pollen-mediated food sensitization, however, in cases such as with almonds and hazelnuts, sensitization to plane tree pollen, birch pollen or mugwort pollen may induce allergies
[52][53] such as those schematically represented in
Figure 2. On the other hand, tree nut allergy cross reaction is highly related to botanical family associations which, for almonds, is common regarding cross-reactivity between other members of the
Rosaceae family
[54][55]. Furthermore, within the
Rosaceae family, a strong source of cross-reaction lies in the structural homology between allergic lipid-transfer proteins (LTP’s). Specifically, in the tree nut group, almond Pru du 3, chestnut Cas s 8, hazelnut Cor a 8 and walnut Jug r 3 are the most predisposed to show cross-reactivity. Besides these, peach Pru p 3 holds higher IgE-binding affinity and a higher number of epitopes compared to other LTP’s, which results in the fact that a peach is a primary sensitizer to LTP’s
[45] and makes it a strong cause for cross-reactivity to other plants, including nuts like almonds
[56]. Other studies performed by Kewalramani et al.
[57] showed extensive IgE cross-reactivity between almonds and apricot seeds, and that there may exist some cross-reactive proteins with pine nut, pecan, walnut, and sunflower seeds.
Figure 2. Most associated allergic cross-reactions with almonds. In orange, allergic cross reactions between almonds and peaches are most commonly due to high structural homology between allergic LTP’s present in the Rosaceae family that both belong to; in blue and grey, it is still unclear if cross reactivity between almonds and other tree nuts groups and peanuts is a consequence of taxonomical proximity and/or high structural homology of IgE-binding epitopes; finally, in green are represented three different pollens which, although it is not usual, when sensitized to them allergies to tree nuts such as almonds could be induced.
2.2. Almond Allergens
To date, ten groups of almond allergens have been identified, namely: Pru du 1, Pru du 2, Pru du 2S albumin, Pru du 3, Pru du 4, Pru du 5, Pru du 6 (amandin), Pru du γ-conglutin, Pru du 8 and Pru du 10. From these groups, only Pru du 1, Pru du 2, Pru du 2S albumin and Pru du γ-conglutin are not included in the WHO-IUIS list of allergens. Their corresponding biochemical names, biological functions, GenBank nucleotides and UniProt annotations, molecular weight, food processing effects and clinical relevance are summarized in Table 2.
Table 2. Almond allergens and their biological function, molecular weight, food processing effects and clinical relevance.
| Allergen |
Biochemical Name |
WHO-IUIS |
Isoallergen and Variants |
GenBank Nucleotide |
UniProt |
Biological Function |
MW (kDa) |
Processing |
Clinical Relevance |
References |
| Pru du 3 |
non-specific Lipid Transfer Protein 1 nsLTP1 |
Yes (2009) |
Pru du 3.0101 |
FJ652103 |
C0L0I5 |
Non-specific lipid transfer protein (nslTP1) and plant defense proteins against pathogens |
9 |
Very resistant to pH, thermal and enzyme treatments |
Systemic and life-threatening symptoms; cross reactivity among Rosaceae fruit |
[58] |
| Pru du 4 |
Profilin |
Yes (2006) |
Pru du 4.0101
Pru du 4.0102 |
AY081850
AY081852 |
Q8GSL5
Q8GSL5 |
Actin-binding protein for cellular function |
14 |
Unstable during heat processing |
Mild symptoms and mainly in oral cavity |
[48] |
| Pru du 5 |
60S acidic ribosomal protein P2 |
Yes (2007) |
Pru du 5.0101 |
DQ836316 |
Q8H2B9 |
Protein synthesis |
10 |
Unknown |
Unknown |
[59] |
| Pru du 6 |
Amandin, 11S globulin legumin-like protein |
Yes (2010) |
Pru du 6.0101
Pru du 6.0201 |
GU059260
GU059261 |
E3SH28
E3SH29 |
Major storage protein |
360 |
Stable to dry heat but can be denatured by boiling |
Severe IgE allergic reactions |
[60] |
| Pru du 8 |
Antimicrobial seed storage protein |
Yes (2018) |
Pru du 8.0101 |
MH922028 |
A0A516F3L2 |
Antimicrobial and seed storage function |
31 |
Unknown |
Unknown |
[61] |
| Pru du 10 |
Mandelonitrile lyase 2 |
Yes (2019) |
Pru du 10.0101 |
AF412329.1 |
Q945K2 |
Highly efficient catalytical enzyme |
60 |
Resistant to enzyme digestion |
Unknown |
[62][63] |
| Pru du γ-conglutin |
Cupin superfamily |
No |
_______ |
_______ |
_______ |
7S vicilin storage protein |
45 for each subunit |
Unknown |
Unknown |
[64] |
| Pru du 1 |
PR-10 protein |
No |
_______ |
_______ |
_______ |
Plant pathogenic and stress response |
17 |
Wet heat processing reduces IgE reactivity |
Unknown |
[65] |
| Pru du 2 |
PR-5/thaumatin-like protein |
No |
_______ |
_______ |
_______ |
Pathogenic response |
23–27 |
Resistant to protease, pH or heat treatment |
Unknown |
[66] |
| Pru 2S albumin |
Prolamin super family |
No |
_______ |
_______ |
_______ |
Seed storage protein |
12 |
Stable to heat treatment |
Unknown |
[64] |
2.2.1. WHO/IUIS Designated Almond Allergens
Pru du 6 (Amandin)
Pru du 6 or amandin is the most well and widely studied almond allergen according to its biochemical function and molecular structure
[67][68][69][70]. It was first reported as an allergen in 1999
[57] but was only recognized in 2010 and added to the WHO-IUIS database.
Biochemically, amandin, also known as almond major protein (AMP), is a member of the cupin superfamily, namely the 11S seed storage globulin family
[31][32]. Globulins are very abundant proteins in legumes and tree nuts, and in almonds they correspond to roughly 65% of total almond protein content
[9].
As an allergen, Pru du 6 have been associated with severe allergic reactions
[69]. Studies on the Pru du 6 isoforms, Pru du 6.01 and Pru du 6.02, showed that the 6.01 isoform is more broadly recognized than the 6.02 isoform. In addition, its denaturation had only slightly effects on IgE-binding intensity in sensitive subjects
[60]. In fact, Pru du 6 polypeptides are highly resistant to heat treatment, which is one of the most common strategies to decrease or even eliminate the allergenic potential of foods. Due to its heat resistance, contamination of food with Pru du 6 polypeptides presents a serious threat to sensitized patients
[71]. On the other hand, some experiments using in vitro models of gastrointestinal digestion suggested that this allergen is sensitive to pepsin but, interestingly, when almond flour is added to other foods, pepsin’s action on Pru du 6 is a lot less effective
[72]. Holden et al.
[73] suggested that the reaction between Pru du 6 and α-conglutin from lupine, another 11S globulin, may be the cause of it.
Pru du 5 (60S Acidic Ribossomal Protein P2)
Pru du 5, also known as 60S acidic ribosomal protein P2, is encoded by
P. dulcis 60S acidic ribosomal protein gene and was included in the WHO/IUIS allergen list in 2007. This name comes from the fact that this allergen is an 11 kDa protein which is a member of the 60S large subunit of the eukaryotic 80S ribosomes
[8], and its biological function is related to protein biosynthesis. Pru du 5 is considered a major almond allergen due to the presence of specific IgE antibodies in 50% of sensitized patients’ sera
[59].
This allergen can exist as a complex with other ribosomal components/proteins or in its free state
[45], with the ability to form homodimers and oligomers
[52][54]. On the allergenicity front, this data is very important because oligomerization gives the allergen the capability of cross-linking IgE antibodies on mast cells and/or basophils surfaces, even if the recognition is made from a single epitope of the allergen
[8].
Although being considered a major allergen and present in the WHO-IUIS allergen list, many authors believe that this classification must be supported by more studies concerning the IgE reactivity of allergic patients’ sera to this allergen
[9][10]. Also, studies regarding the biochemical and immunological properties of Pru du 5 in its natural state as an allergen are lacking
[8], leading to the conclusion that newer and tougher studies are needed.
Pru du 3 (nsLTP)
Added to the WHO/IUIS database in 2009, Pru du 3 is a non-specific lipid transfer protein 1 (nsLTP1) belonging to the subfamily of nonspecific lipid transfer proteins (nsLTPs)
[55]. This family includes proteins constituted by a hydrophobic core to ease lipid transference such as phospholipids, steroids, fatty acids, and glycolipids between membranes. Besides that, nsLTPs are also known as pathogenesis-related 14 (PR-14) proteins, a member of the prolamin superfamily
[9][45], which actively participate in plant-defense mechanisms against fungal and bacterial pathogens and other environmental stresses
[56].
In almonds we identified and characterized three nsLTPs
[62] with identical molecular weights (9 kDa) and similar amino acid lengths: 117, 123 and 116 amino acids for Pru du 3.01, 3.02 and 3.03, respectively. In the three isoallergens, there are eight cysteine conserved residues, which allow the formation of four disulfide bonds
[9].
Due to the typical accumulation of this protein family in outer epidermal layers, the peels are associated with stronger allergenicity compared with the pulps of the fruits in the
Rosaceae family. Regarding allergenicity, this protein family is quite concerning because of its resistance to abrupt pH changes, pepsin digestion, thermal treatments, and the ability of restore folding structures and the consequent proprieties after cooling
[74]. Cross-reactivity is also a major concern once the nsLTP family is characterized by a high level of conserved sequences and tridimensional structures allowing IgE recognition, which in turn results in cross-reactivity between species
[56]. Furthermore, the Rosaceae fruits and seeds normally present nsLTP proteins, and with that comes a high probability of cross-reactivity between, for example, apples, peaches, cherries, apricots and almonds
[63]. This latest evidence is the main reason why nsLTPs are included in the panallergens group—allergens ubiquitously spread throughout nature, showing a high level of conservation besides being from different and unrelated organisms
[8].
Pru du 4 (Profilins)
Pru du 4 proteins are included in the profilin family and are encoded by the putative genes
Pru du 4.01 and
Pru du 4.02 [48] which, although present in different size fragments (1041 and 754 bp, respectively) encode two proteins with similar sequences (131 aa), molecular weights (roughly 14 kDa) and acidic properties (
pI near 4.6)
[9].
These proteins can establish high-affinity complexes with monomeric actin, leading to its polymerization into filaments. Once they are associated with actin, it is not surprising that profilin allergens are included in the panallergens group with Pru p 4.01 and Pru av 4 from peaches and sweet cherries, respectively, being the most similar and identical proteins (99 and 98%, respectively) in relation to almond profilins. In general, profilins seem to present moderate structural stability, and harsh conditions contribute to their denaturation and consequent loss of conformational structure. In almonds, Pru du 4 profilins are very difficult to detect by immunoblot screens because of their low levels and their labile character. Because almond profilins antibodies are detected in 44% of patients’ sera, they are classified as minor allergens
[48].
Pru du 8
Pru du 8 is one of the latest allergens included in the WHO-IUIS database. This allergen was reactive in six of eighteen sera of almond allergic patients
[10][72]. Biochemically speaking, Pru du 8 is characterized by a signature repeat of a CX
3CX
10-12CX
3C (X being any amino acid), motif which is also related to the N-terminal or the signal peptide of some vicilins
[61], and it was also reported to maintain antimicrobial function of some peptides derived from macadamia vicilin
[75].
The first nomenclature attempt for this allergen was based on the sequencing of two short peptides of this allergen to reveal the identity of an IgE-reacting protein several years ago. Nevertheless, the result was a misidentification of this allergen as an almond 2S albumin because of the sequence alignment of the two peptide sequences and those in other 2S albumin proteins
[64]. More recently, in silico investigations and bioinformatic analyses reopened the debate, naming this allergen as Pru du vicilin (almond 7S vicilin), although some authors believe in a second misidentification
[8][76]. In fact, the authors claim that this misidentification is due to the similarity between the signal peptides of vicilins of other species and Pru du 8. Besides that, it is argued that some Pru du 8 orthologs present in the NCBI database, most of them predicted by automatic genome annotations, are incorrectly named as vicilin-like proteins due to the absence of the cupin signature domains of 7S vicilins
[8][61].
All this controversy shows that further studies are needed to better elucidate the actual protein family of Pru du 8.
Pru du 10
To date, this allergen was the last one to be added to the WHO-IUIS database. This allergen corresponds to mandelonitrile lyase 2 (formerly hydroxynitrile lyase 2), which is a highly effective catalytic enzyme
[62]. This allergenicity was recognized after allergic response to almond ingestion where thirteen of eighteen almond allergic patients were sensitized. Also, the Pru du 10.0101 isoallergen was identified and added to the WHO-IUIS allergen information.
Besides being identified in raw almond samples, this protein was also identified in digested samples, which may indicate that this allergen is able to overcome the digestion process
[63]. Still, there is a lack of information regarding this allergen which clearly shows that more studies should address this issue.
2.2.2. Allergens Not Included in the WHO/IUIS Allergen List
There are two main processes to classify a protein as a food allergen, based on immunological data such as the IgE reactivity or based on sequence similarity with proteins of other species already considered allergens. For an allergen to be included in the WHO-IUIS database, immunological data is required and because of that, some authors defend that those which cannot be supported by it should hardly be assumed as an allergen. However, bioinformatic-based investigation is very important to promote further investigation and make aware the scientific and industrial community to the dangers of food allergens.
Pru du γ-Conglutin
The IgE and serological reactivity to Pru du γ-conglutins were not associated with any clinical symptoms and because of that, they are not recognized into standard clinical nomenclature
[10].
After the report and characterization of conglutins in other fruits and seeds such as lupine
[77], peanut
[78], soybean
[79] or cashew
[80], in almonds an N-terminal peptide sequence of 25 aa belonging to a IgE binding protein with a molecular weight of 45 kDa was also identified, presenting around a 40% identity rate between the mature forms of γ-conglutin from wide and narrow-leafed lupine
[64]. Moreover, with a high similarity, approximately 50%, between this almond protein and 7S globulin from soybean, this allergen was considered a vicilin (7S globulins) of the cupin superfamily
[8][9]. Nevertheless, some authors do not agree with this classification, stating that γ-conglutin is not a vicilin due to its biochemical properties
[8]. In particular γ-conglutin presents sequence and structural similarities with xyloglucan-specific endo-beta 1,4-glucanase inhibitors, however such glucanase inhibition properties are not related to the natural γ-conglutin due to is peptidase cleavage susceptibility
[81].
The same authors believe that more studies regarding immunological and biochemical properties of this protein are needed, and the confirmation of this assumption would make this protein the first food allergen from this supposed protein family.
Pru du 2 (PR-5/Thaumatin-Like Protein)
This allergen group is also known as PR-5 or thaumatin-like proteins (TLPs) and are responsible for the biological response to pathogen infection, fungal proteins, and osmotic stress. The TLP’s group is known to be very resistant to proteases, heat-induced denaturation, and pH variations, possibly because of sixteen conserved cysteine residues which form eight disulfide bonds
[63]. Several isoallergen genes have been identified which code for TLP, ranging in molecular weight from 23 to 27 kDa. Also, the isoallergens aminoacidic sequence length ranges from 246 aa to 330
[84].
Like PR-10 proteins, no immunological characterization of PR-5 almond proteins exists. Although, it is believed that these proteins are almond allergens due to the high sequence identity with Pru p 2, a peach allergen
[85]. Moreover, due to their biochemical properties, traditional food-processing practices do not significantly influence these protein’s structure and characteristics, so they could affect sensitive patients
[9].
Pru du 2S Albumin
Included in the prolamin superfamily, 2S albumins are an important group of seed storage proteins involved in seed growth and in defense related mechanisms
[86][87]. Besides 2S albumin, the prolamin superfamily also includes other protein groups such as the nonspecific lipid transfer proteins (nsLTPs), prolamin storage proteins and α-amylase/trypsin inhibitors, which may indicate several cross-reactions
[88].
2S albumins are thought to be somehow resistant to acidic pH enzyme digestion, particularly the albumins with proteolytic activity and surfactant denaturation effects. These conclusions come from the fact that is believed to this proteins cause sensitization along the intestinal tract, which could only be possible if the previous resistances were actually accurate
[89].
As an allergen, the strongest data that lead to the classification of almond 2S albumins as almond allergens is the two short partial peptide sequences with high similarity with 2S albumins of other species
[90] that, as discussed in
Section 3.2.1, some authors believe to be a misidentification and really correspond to Pru du 8 proteins
[8]. In fact, 2S albumins of other species, such as Ara h 2 (peanut 2S albumins) for example, are very potent allergens
[91][92][93] and for this reason the assessment of whether these almond proteins are allergens or not is required and imperative.
2.3. Methods for Almond Allergens Detection
Most of the methods used for the detection of almond allergens are based in immunochemical properties, DNA techniques and, lately, in Mass Spectrometry (MS) approaches
[9].
The immunochemical methods are based on the interaction between immunoglobulins and epitopes present in the target protein. For almond allergen detection, lateral flow devices (LFD), immunoblotting and especially Enzyme-Linked Immunosorbent Assay (ELISA), are very standard methods and the usual techniques for quantitative and qualitative detection of food allergens
[94][95]. This comes from the fact that ELISA tests, for example, have enough sensitiveness for protein detection (in the orders of ppm), being the main advantage of the fast assessment, which is important for clinical purposes
[95]. Several immunological commercial kits, such as the ones exemplified in
Table 3, have been developed with the objective of delivering the most sensitive result in the shortest amount of time. As seen in the kit’s characteristics, ELISA-based methods provide more sensitive results, as their limit of detection is lower than the LFD-based kits. However, the assay time is longer for the ELISA cases. Taking this into consideration, the assay type should be taken into serious consideration, according to the situation that are supposed to be used.
Table 3. Example of commercial immunological kits for almond detection and/or quantification and their main characteristics: time for results including extraction times, assay type, limit of detection (LOD), limit of quantification (LOQ) and their manufacturers.
| Kit 1 |
Assay Time |
Assay Type |
LOD (ppm) |
LOQ
(ppm) |
Company |
| ELISA-based |
|
|
|
|
|
| MonoTrace ELISA kit |
40 min |
Monoclonal antibody-based ELISA |
0.15 |
1 |
BioFront Technologies, Tallahassee, FL, USA |
| SENSISpec ELISA almond |
75 min |
Sandwich enzyme immunoassay |
0.2 |
0.4 |
Eurofins Technologies, Budapest, Hungary |
RIDASCREEN FAST
Mandel/Almond |
50 min |
Polyclonal antibody specifically for almond protein
detection, sandwich ELISA |
0.1 |
2.5 |
R-Biopharm AG, Madrid, Spain |
| AgraQuant® Plus Almond |
30 min |
Sandwich enzyme-linked immunosorbent assay |
0.5 |
1 |
Romer Labs®, Getzersdorf, Austria |
| LFD-based |
|
|
|
|
|
| AgraStrip® Almond |
11 min |
Lateral flow device |
2 |
__________ |
Romer Labs®, Getzersdorf, Austria |
| Reveal 3-D Almond Test |
10 min |
Lateral flow device |
5 |
__________ |
Neogen Corp., Lansing, MI, USA |
| Lateral Flow Almond incl. Hook Line 2 |
10 min |
Lateral flow device |
1 |
__________ |
R-Biopharm AG, Madrid, Spain |
Another possible approach, instead of looking directly for the protein itself, is the DNA-based method where an amplification is performed of the gene fragment responsible for encoding the allergen by Polymerase Chain Reaction (PCR), allowing quantitative and qualitative measurement using real-time PCR or endpoint PCR assays, respectively
[10]. One of the advantages of these methods is that they rely on the detection of low quantities of almond DNA even after food processing, which could promote the degradation of some allergen proteins and therefore not be detected by immunological approaches
[96]. However, the presence of the gene encoding the allergens does not imply its expression and, because of that, the synergistically use of DNA-based techniques and ELISA could overcome some of the drawbacks of both techniques
[97].
Proteomics play a very important role in the food allergy problematic, firstly on a fundamental investigation basis to characterize allergens and further to their application in the diagnostic routines. Namely, a variety of tests and methods must be applied to characterize allergens according to their allergenic activities, purity and folding properties. Following this line of thought, SDS-Polyacrylamide Gel Electrophoresis (SDS-PAGE) is a reliable technique to determine purity, and following 2 Dimension (2D) electrophoresis, capillary electrophoresis or High-Performance Liquid Chromatography (HPLC) are great techniques to access individual isoforms and obtain more additional information in general. Further, MS techniques are powerful tools to determine protein molecular masses, being Matrix Assisted Laser Desorption Ionization (MALDI) and ElectroSpray Ionization (ESI), as the most commonly used
[98][99]. MS techniques have been the most recent methods to be explored for qualitative and quantitative purposes
[100][101]. For example, the isolation and characterization of Pru du 3 allergen was conducted using MS techniques where the full sequence was obtained by Liquid Chromatography ElectroSpray Ionization Orbitrap Mass Spectrometry (LC-ESI-Orbitrap-MS)
[102]. Mass spectrometry has the advantage of ELISA tests which can directly identify proteins with a high sensitivity, and therefore could provide a direct risk evaluation and, besides that, can be used for the detection of multiple allergens simultaneously
[102]. MS could be the chosen technique for a standard test; however, it is a relatively recent approach which demands expensive equipment and specialized personnel. At this standpoint, further improvements are required to allow easier access and profitable use by clinical facilities
[10].
Another methodology under development is based on microarrays. Namely, allergen microarrays such as the MeDALL allergen-chip have been explored for the diagnosis and monitoring of allergies. The main advantages rely on the simultaneous detection of several allergens with a minimal amount of sera in a reduced time. The development of this chip has the purpose of monitoring IgE and IgG reactivity profiles against 170 allergens in sera collected from European birth cohorts. With that information, it would be possible to make a geographical association of clinical important allergens in different populations and track the progress of food allergy itself and would allow clinical therapies to act in a prophylactic and more personalized manner
[103].
It is worth mentioning the basophil activation test (BAT) as a powerful method for tree nut allergy diagnosing
[104]. This is an in vitro assay based on flowcytometry protocols that, essentially, allows the evaluation of activation and/or degradation levels of basophils upon the intentional contact with the pretended food allergens
[105]. However, it also has some limitations, mainly because of the level of equipment required which makes difficult the use of this technique in small medical centers; this could be overcome with the use of specialized centers and with new research to lower the costs. On other hand, results have been shown that BAT assays have very strong performances and useful results, including multi-nut sensitizations and, because of that, medical infrastructures should take this test into consideration for these kinds of diagnostics
[106].