1000/1000
Hot
Most Recent

Poor semen quality and abnormal sperm structure or function by and large constitute a predominant presentation of male infertility and are among the major reasons why couples seek the services of assisted reproduction. Natural biomolecules, such as polyphenols or flavonoids have garnered substantial attention from the scientific community as well as public media. In the meantime, it has been suggested that administration of natural biomolecules has been shown to have a positive impact male infertility. A number of in vivo as well as in vitro studies have reported that active components isolated from natural resources could enhance blood circulation in the male reproductive system and support the synthesis and secretion of androgens. Animal and human reports also suggest that natural biomolecules may play important roles in the enhancement of semen quality, including sperm concentration, motility, vitality, and structural integrity.
Resveratrol (3,5,40-trihydroxistilbene; RES) is a polyphenol found in a wide array of dietary sources such as grapes, peanuts, berries, pistachios, plums, and red wine. The molecule is a phytoalexin, as its inherent function is to offer protection to the producer against exogenous stress factors (UV radiation, ozone, injury, or fungal infection). Because of its structural similarities with estradiol or diethylstilbestrol and its ability to modulate estrogen-sensitive systems, RES is defined as a phytoestrogen [1][2].
Being probably the most studied biomolecule of recent decades, RES has attracted widespread scientific attention because of the “French paradox” hypothesis, which associates low incidence of cardiovascular diseases and a long-life expectancy of French people to a moderate consumption of red wine despite a diet containing meals with high amounts of saturated fat [3][1][2].
Currently available literature suggests that RES presents with a wide array of properties that may be useful in the prevention and improvement of a variety of health issues including obesity, diabetes, atherosclerosis, hypertension, malignant and neurodegenerative diseases. Furthermore, the biomolecule presents with anti-inflammatory, antimicrobial, anti-aging, and antioxidant effects [1][2]. Resveratrol is also the most potent bioactive substance that activates sirtuin 1 (SIRT 1), the most-conserved mammalian NAD+-dependent protein, which may account for its numerous metabolic benefits in animals as well as in humans [4].
In recent years, numerous studies have focused on the effects of RES on male reproductive performance and reported that RES enhances spermatogenesis by stimulating the hypothalamic-pituitary-gonadal axis, triggers penile erection, reinforces testosterone production, increases testicular sperm count and epididymal sperm motility [3][5]. Furthermore, it was suggested that RES administered in vivo or in vitro acts as an effective reactive oxygen species (ROS)-quencher and stabilizes the antioxidant balance of male reproductive cells and tissues. Moreover, first clinical studies examining the impact of RES-fortified nutraceuticals are currently underway [6]. As such, it may be hypothesized that RES presents with a complex nature and affects multiple cellular and molecular targets within the male reproductive system (Figure 1).
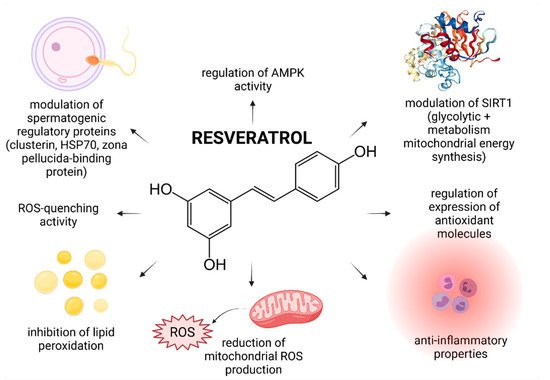
Figure 1. Most frequently reported beneficial effects of resveratrol on spermatogenesis, sperm structural integrity and functional activity.
The first clinical trial that focused on the effects of RES on a possible improvement of male idiopathic infertility was published only recently by Illiano et al. [6]. In this prospective single-center clinical trial, patients diagnosed with oligozoospermia and/or asthenozoospermia were administered with a nutritional supplement containing 150 mg RES together with vitamins D, B6, B12 and folic acid for 6 months. The study found that while the sperm morphology, seminal pH and volume did not change during the treatment period, consumption of the supplement led to a significant improvement of the sperm concentration and motility. The authors hypothesize that this phenomenon may be primarily attributed to the ability of RES to modulate SIRT1 that controls cellular pathways crucial for glycolysis and mitochondrial energy metabolism–respiratory balance, necessary for a proper testicular function. Furthermore, it was suggested that RES stabilizes the mitochondrial genome, and aids to prevent mitochondrial DNA (mtDNA) defects, which are commonly observed in infertile patients.
While the exact role of RES in male reproduction is still not completely understood, a substantial number of animal studies indicates that the biomolecule is able to pass through the blood-testis barrier, imparting its protective effects on the testes, and subsequently on the sperm quality [5].
A solid foundation for posterior investigations of the impact of RES on male reproduction was established Juan et al. [7] according to who administration of 20 mg/kg/day RES led to an increased diameter of the seminiferous tubules, accompanied by greater sperm counts in healthy rats. Since other sperm quality parameters were not affected, the authors conclude that RES did not exhibit any toxic effects on the animals and enhanced the sperm production primarily by stimulating the hypothalamic-pituitary-gonadal axis.
Subsequently, a large quantity of reports emerged, emphasizing on ameliorative and stimulating effects of RES on the process of spermatogenesis and subsequent sperm quality that may be compromised by diseases, stress, lifestyle changes, medication, or environmental risk factors.
The effect of RES on the sperm motion and testicular oxidative profile in rats presenting with triiodothyronine-induced hyperthyroidism was investigated by Ourique et al. [8]. It was revealed that RES treatment (1 mg/kg/day or 10 mg/kg/day) counteracted a decline of sperm motility, which was accompanied by a significant prevention of lipid peroxidation (LPO) and a stabilization of catalase (CAT) and glutathione peroxidase (GPx) activities. This behavior of RES may be attributed to its exceptional antioxidant properties and ability to enhance the activity of key antioxidant enzymes. Positive effects of RES have also been studied in metabolic disorders such as diabetes. Abdelali et al. [9] demonstrated that RES ameliorated Type 1 diabetes mellitus-induced abnormal sperm formation, oxidative damage, sperm DNA fragmentation, and furthermore affected the polyADP-ribose polymerase (PARP) signaling pathway in the rat testes. The biomolecule proved to be equally effective in Type 2 diabetes, as a dose of 1.5 mg/kg RES increased the sperm count, motility and viability, and furthermore provided a significant protection to the sperm chromatin integrity and stability in streptozotocin (STZ)-nicotinamide-treated rats [10].
Possible ameliorative effects of RES on the spermatogenic dysfunction as a result of high-intensity exercise were studied by Guo et al. [11]. This study revealed that rats subjected to 9 weeks of intensive exercise and 50 mg/kg/day RES, presented with a stable sperm density. Furthermore, the concentrations of interleukin 6 (IL-6) and tumor necrosis factor α (TNF-α) were found to be significantly decreased, suggesting potent anti-inflammatory properties of RES. Using a global proteomic approach, the authors observed, that RES was able to modulate the expression levels of key of spermatogenic regulatory proteins, including Clusterin, Zona pellucida binding protein (Zpbp), Heat shock proteins and Centrin 1, indicating that RES could be indeed able to interact with molecular pathways crucial for a proper sperm production and function.
A significant impact of RES on the molecular machinery of the sperm production, maturation and vitality was reported by Shati et al. [12], who investigated possible protective roles of RES against cisplatin (Cis)-induced reproductive dysfunction in rats. It was revealed that 20 mg/kg RES administration post-Cis therapy restored all sperm parameters and prevented testicular apoptosis mediated by inhibition of key apoptotic markers, including cleaved caspase-3, p53, calpain-1/cleaved caspase-12, or p-ERK1/2, while the levels of p-Akt/p-Bad were upregulated following exposure to RES. Molecular changes in the testicular tissue were translated into a significant preservation of the sperm count, motility and morphology, a reduction of testicular apoptosis and a stabilization of the antioxidant molecules in the testes.
An inhibition of germ cell apoptosis as a result of RES supplementation was also reported in New Zealand white rabbits intoxicated with codeine. At the same time, it was observed that RES exhibited strong sperm DNA-protecting properties, as evidenced by a reduced 8-hydroxy-2’-deoxyguanosine (8-OHdG) production, which is acknowledged as a biomarker of oxidative DNA damage [13]. In the meantime, an improved histological structure of testes, accompanied by an increased sperm motility, count and viability in mice exposed to RES (2, 8 or 20 mg/kg/day) and 20 mg/kg/day morphine was reported by Jalili et al. [14]. A significant ability of RES to inhibit nitric oxidase and thus to decrease nitric oxide production was observed as well. In a later study, Jalili et al. [15] reported that RES (2, 8 or 20 mg/kg) was able to stabilize the testicular redox balance and prevent LPO in rats exposed to malathion—a highly toxic and prooxidant organophosphate.
Currently available in vitro data suggest that RES is accompanied by a peculiarity; at low doses, the biomolecule improves cell survival and vitality, while at high doses, it exhibits cytotoxic effects [16]. One of the first studies emphasizing on this dichotomy in spermatozoa was published by Collodel et al. [17]. The authors evaluated the impact of different RES concentrations (6–100 µmol/L) on swim-up human spermatozoa and noted that the best beneficial effect was reached at 6 and 15 µmol/L RES, while a complete inhibition of sperm motility was observed at 100 µmol/L RES. Similar data were observed in the case of bovine spermatozoa [18]. Interestingly, Shabani Nashtaei et al. [19] observed that while the highest RES concentrations (25 mmol/L) significantly reduced total and progressive human sperm motility, secondary kinematic parameters, such as curvilinear velocity, average path velocity and straightness were not affected. Finally, Cui et al. [20] pointed out to a significant improvement in the motion behavior and acrosin activity of spermatozoa collected from obese patients with astenozoospermia that were incubated for 30 min. in the presence of 30 µmol/L RES.
RES has been proven as an exceptional in vitro antioxidant that may be attributed to four main mechanisms of action: (a) a direct prevention of ROS overproduction in the sperm mitochondria, (b) the ability to scavenge superoxide, hydroxyl radical, and metal-induced radicals, (c) inhibition of oxidative insults to the sperm lipids, and (d) regulation of the activity of endogenous antioxidants [1][2][5][21].
Alamo et al. [22] investigated the impact of RES on human sperm damage caused by benzo-a-pyrene (BaP), a polycyclic aromatic hydrocarbon which is a potent prooxidant and promoter of caspase as well as endonuclease activation. The authors observed that 15 µmol/mL RES significantly counteracted the detrimental effects of BaP on the sperm chromatin compactness and oxidative damage to the lipids. Moreover, Tvrdá et al. [23] reported that particularly 50 µmol/L RES maintained the sperm motion and viability, stabilized the activities of antioxidant enzymes and prevented LPO in bovine spermatozoa subjected to oxidative stress induced by ferrous ascorbate.
According to Collodel et al. [9] 15 µmol/L RES preserved the chromatin texture of spermatozoa exposed to tert-butyl hydroperoxide (TBHP) which fortifies a possible utilization of RES as a supplement during cryopreservation or in vitro fertilization—intracytoplasmic sperm injection (IVF-ICSI). This potential was later confirmed by Li et al. [24] according to who the presence of 10−4 mol/L RES in the washing and fertilization medium significantly decreased ROS production, LPO, phosphatidylserine externalization, and protected the mitochondrial function as well as acrosomal integrity of sex-sorted bull spermatozoa, thereby increasing the blastocyst percentage and quality following IVF.
Accordingly, RES has been extensively studied as a suitable alternative supplement to cryopreservation media, primarily thanks to its ability to prevent oxidative damage to biomolecules critical for the sperm survival. An improved post thaw sperm motility, membrane stability and mitochondrial activity has been reported in human [25], bovine [26], ram [27] and boar spermatozoa [28] exposed to RES during the freezing and thawing process. Nevertheless, dose-dependent effects of RES have been frequently observed during sperm cryopreservation as well.
Branco et al. [29] reported that while 10 mmol/L RES significantly decreased DNA damage in fertile as well as infertile men, the compound was not able to prevent a post-freezing motility decline. This inability to prevent the loss of motility was observed in later studies [15][30] despite RES being effective in protecting the sperm lipids against cryodamage.
The hypothesis that RES may modulate the activity of AMP-activated protein kinase (AMPK) was postulated by Shabani Nashtaei et al. [19] who reported that 5, 15 or 25 µmol/L RES significantly increased AMPK phosphorylation, as opposed to Compound C—a known AMPK inhibitor. The exact mechanism by which this occurs however needs to be clarified further, since AMPK activation can occur through an array of mechanisms, which may include an increase in the AMP/ATP ratio, inhibition of mitochondrial ATP synthase or ROS overproduction [31][32].
Lastly, it was suggested that RES may be able to affect key paternal transcripts considered as potential markers for male fertility (protamine 1 and 2) and pregnancy success (adducin 1 alpha) in cryopreserved human spermatozoa, particularly by AMPK stimulation and improvement of interactions among mRNAs, making them more resistant to cryopreservation [33].
Summarizing the collected information, RES seems to be a complex biomolecule, able to modulate male reproductive performance at the tissue, cellular as well as molecular level. Its complexity and ability to interact with a wide array of biomolecules and signaling pathways fortify it potential as an in vivo or in vitro supplement for the prevention or management of male subfertility. Nevertheless, the existence of unclear and often contradictory data suggests that further studies are necessary to consolidate the understanding of the properties of RES and its roles in male reproduction.
Quercetin (3,30,40,5,7-pentahydroxylflavone; QUE) is a flavonol-type flavonoid which may be found in citrus fruits, berries, herbs and spices, red wine, cocoa, tea and fruit juices [34]. The biomolecule has been reported to exhibit anti-inflammatory, anticarcinogenic, antibacterial, anti-aggregatory and antidiabetic effects [34][35]. Within the flavonoids, QUE is considered to be the most potent scavenger of ROS and nitric oxide [36]. These antioxidant properties could be attributed to its direct ROS and reactive nitrogen species (RNS)-trapping properties, ability to chelate metals, and to interact with lipid bilayers, modulate enzymes and/or induce repair mechanisms. Moreover, QUE has been reported to substantially fortify the endogenous antioxidant defense mechanisms because of its contribution to the total antioxidant capacity. Additionally, QUE may be effective because of its ability to interact with and penetrate through lipid bilayers [37].
Evidence on the impact of QUE on male reproduction is still controversial and inconsistent. While most studies emphasize on stimulating and protective effects of QUE on male reproductive performance, there is still a solid number of reports which indicate its potential toxic or adverse effects on reproductive cells and tissues. This controversy is primarily caused by a lack of understanding on the exact bioavailability and behavior of QUE in living tissues. Furthermore, as opposed to RES or lycopene, human trials have not been taken up yet. By and large, in vivo animal studies indicate beneficial effects of QUE on the testicular function (Figure 2).

Figure 2. Most frequently reported beneficial effects of quercetin on spermatogenesis, sperm structural integrity and functional activity.
The effects of QUE on the testicular structure and semen quality depending on its dose and time of administration have been studied by Taepongsorat et al. [38]. Male rats were injected with 0, 30, 90 or 270 mg/kg b.w./day QUE and its impact was assessed following 3, 7 and 14 days of treatment. The shortest time of QUE treatment had no effects neither on the weights of the reproductive organs nor on the sperm quality. Nevertheless, a significantly increased testicular and epididymal weight, accompanied by an increased sperm motility, viability and concentration was observed following administration of 90, or 270 mg/kg b.w./day QUE for 7 and 14 days, respectively. The authors speculate that QUE may affect the sperm quality indirectly, either through a stimulation of other sex organs or via an enhancement of the hypothalamic-pituitary-testicular axis. A direct effect on the sperm quality was not proposed, as the observed changes might have been attributed to sperm retention in the epididymis and epididymal lumen dilatation, leading to an increased quality of the stored sperm reserve.
According to Yelumalai et al. [39] STZ-nicotinamide induced male diabetic rats treated orally with QUE (10, 25 and 50 mg/kg/b.w.) presented with an increased sperm count and motility, viability and membrane integrity while the number of spermatozoa with abnormal morphology decreased. Furthermore, the activities and mRNA expression levels of major antioxidant enzymes (superoxide dismutase—SOD, CAT, GPx) increased while LPO, NF-κβ and TNF-α levels decreased, suggesting strong antioxidant and anti-inflammatory properties of QUE in counteracting reproductive complications resulting from diabetes.
Furthermore, it was reported that QUE (30 mg/kg) administered orally improved the epididymal weight and testicular length, stabilized the architecture of seminiferous tubules and decreased the incidence of testicular apoptosis in rabbits subjected to induced heat stress. A significant improvement was also observed in the epididymal sperm motility and kinetics, viability, mitochondrial activity, and acrosome integrity. It was postulated that QUE affects the sperm and testicular configuration while minimizing oxidative insults, which in turn protects the testes and spermatozoa against heat stress-induced damage [40].
QUE proved to be a highly effective biomolecule in toxicological studies as well. Jahan et al. [41] observed that in rats intoxicated with arsenic QUE treatment (50 mg/kg) significantly prevented tissue deposition of the heavy metal within the testis, as revealed by an improved testicular structure and a significantly higher daily sperm production. Sperm DNA damage, induced by arsenic, was also significantly reversed. Furthermore, Ben Abdallah et al. [42] reported that administration of 50 mg/kg/day QUE along with lambda-cyhalothrin (LTC) significantly prevented a decrease in functional sperm parameters and LPO products while increasing the testicular activities of antioxidant enzymes as well as glutathione (GSH) concentration. Bu et al. [43] noted that QUE supplementation (100 mg/kg) significantly restored the depletion level of GSH and the activities of SOD and GPx in mouse germ cells intoxicated with 3-methyl-4-nitrophenol from diesel exhaust particles.
As discussed earlier, we must acknowledge that in case of a broader concentration range, QUE may act dose dependently as either a stimulant at low doses or as an inhibitor at high doses [37]. The flagship study on this issue was published by Ranawat [44] who assessed the effect of intraperitoneal QUE administration (2, 8 and 20 mg/kg) on male reproductive function in adult mouse for two weeks. QUE increased the generation of ROS and LPO in the testes with a concomitant decrease of the sperm count and motility in a dose dependent manner. Activities of SOD and CAT as well as the levels of GSH were found to be decreased in a dose dependent manner. Testicular histomorphology was also altered, and a significant loss of various germ cell populations was observed depending on the dose of QUE applied. This discrepancy was fortified by Aravindakshan et al. [45] who showed that administration of a higher QUE dose (300 mg/kg b.w.) reduced the fertility rate of male rats during the first two matings, however the reproductive function was recovered several weeks post-treatment.
This controversy may be explained by the fact that QUE may undergo a process of oxidation, while its oxidized form will arylate GSH and protein thiol groups, ultimately leading to its consumption. Low GSH concentrations will subsequently increase cytosolic calcium (Ca2+) concentration and leakage of lactate dehydrogenase (LDH) [46]. If the cytosolic free calcium levels trespass their physiological limits, excessive Ca2+ may either cause cytotoxicity or trigger programmed cell death [47]. This sequence of intracellular events will ultimately lead to an inhibition of sperm motility and germ cell depletion even though initially QUE acts as an antioxidant. As such, doses of the biomolecule must be considered carefully, in order not to trespass the delicate threshold between its beneficial and toxic effects on the male reproductive system.
In the meantime, most in vitro studies on QUE as an alternative supplement for sperm processing, culture and preservation indicate that the biomolecule has a stimulating effect on the structural integrity and functional activity of the male gamete.
According to Diao et al. [48], 10 μmol/L QUE could significantly improve the motion behavior of spermatozoa collected from leukocytospermic patients. In vitro studies on animal spermatozoa also agree that exposure of male gametes to particularly QUE concentrations ranging between 10 and 25 μmol/L may lead to a higher preservation of motility in bovine [49] and boar spermatozoa [50][51]. Furthermore, pivotal experiments on cryopreserved boar [52], stallion [53], red fowl [54] and ram [55] spermatozoa suggest that low QUE concentrations administered to the freezing and thawing medium improve the sperm motility and a subsequent fertilization potential.
Furthermore, El-Khawagah et al. [56] observed that besides overall motility, 10 μmol/L QUE positively affected secondary kinematic parameters of buffalo bull spermatozoa, including their progressive motility, curvilinear velocity (VCL) and amplitude of lateral head displacement (ALH). Similar effects of QUE on the sperm velocity parameters were reported in studies on frozen-thawed stallion [53] and boar [52] spermatozoa, and indicated a positive correlation between QUE supplementation, sperm fertilizing capacity, in vitro fertilization success and pregnancy rates.
Several explanations are offered to an increased sperm motion behavior following in vitro QUE administration. It was previously suggested that QUE could modulate the activity of CatSper channels which mediate progesterone induced Ca2+ influx and play a role in the sperm hyperactivation and acrosome reaction [57]. Furthermore, it may be feasible to assume that the antioxidant properties of QUE enable the molecule to further stabilize progesterone receptors on the sperm membrane [48]. Beneficial effects of QUE on the sperm motion may be furthermore related to its interaction with Ca2+-ATPase, a key enzyme involved in supporting sperm motility, possibly by its role in intracellular cyclic adenosine monophosphate (cAMP) production [58].
Recent in vitro studies indicate that QUE is a highly efficient biomolecule in preventing the loss of the mitochondrial membrane potential [50] and a subsequent mitochondrial dysfunction by its ability to accumulate inside the mitochondria [59] and to control ROS production by its antioxidant activity [56]. The mitochondrial system is considered to be the primary source of intracellular ROS, whereas QUE may play important roles in the absorption and neutralization of ROS created mainly by the activity of NADPH oxidase and NADH-dependent oxidoreductase, localized in the inner mitochondrial membranes [34][35][60]. Furthermore, it was revealed that QUE treatment led to a higher stabilization of the mitochondrial genome as evidenced by a significantly decreased sperm mtDNA copy number in leukocytospermic semen samples [48]. Correspondingly, the levels of cytochrome B, NADH 5 [48] and succinate dehydrogenase were significantly increased after treatment with QUE, suggesting a protective effect of the biomolecule on important components of the mitochondrial respiratory chain.
Protective effects of QUE on the mitochondrial structure and function, coupled with its straight-forward antioxidant properties could furthermore decrease possible ROS leakage from the sperm mitochondria to the nucleus, minimizing the susceptibility of the male genome to oxidative insults. Correspondingly, a significantly reduced DNA fragmentation following in vitro exposure to QUE was found in bovine [61], stallion [62] and boar spermatozoa [50]. Moreover, it was suggested that 30 µmol/L QUE could prevent chromatin distortion caused by the exposure of human spermatozoa to tert-butylhydroperoxide (TBHP) [63].
The ability of QUE to prevent or counteract ROS overproduction has been acknowledged in numerous reports. According to Tvrda et al. [49][50][61] QUE was highly efficient in opposing high levels of superoxide, which is considered to be the prevalent ROS produced by the sperm cell, and the first one to initiate the Fenton reaction. Further reports on the effects of QUE on human [63], bovine [64] and rat [11] spermatozoa speculate that QUE could exhibit its superoxide trapping properties through the inhibition of NADPH oxidase and/or NADH-dependent oxidoreductase; SOD mimicking; or direct superoxide quenching. Hence, it may be suggested that the biomolecule may be particularly efficient during the initiation of oxidative chain reactions, keeping superoxide in physiological levels.
This property of QUE also enables the biomolecule to prevent further increase of hydrogen peroxide (H2O2) production, which may result in LPO. As such, a significantly decreased concentration of malondialdehyde (MDA) in spermatozoa exposed to QUE is a frequently observed phenomenon, as reported in humans [63], goats [65], bulls [49][56], boars [50], and stallions [66].
Despite a convincing body of evidence on the protective effects of QUE on male gametes, the quercetin paradox has been often observed in vitro as well, particularly in cases when high doses of QUE were supplemented [37][46].
An in vitro study carried out on human semen samples showed that treatment with 50–100 mmol/L QUE resulted in an irreversible and dose-dependent sperm motility inhibition [67]. A disruption of sperm motion and viability was observed in the case of bull [49][61] and boar [50] spermatozoa as well. A decreased total and progressive sperm motility, velocity, wobble, oscillation index and a lower percentage of fast cells were reported by Silva et al. [68] and Borges et al. [69] who studied the effects of QUE on frozen goat spermatozoa.
Adverse effects of QUE on the sperm motion behavior may be directly associated with the previously discussed ability of the biomolecule to modulate Ca2+-ATPase activity [70]. Inappropriately high doses of QUE may decrease the activity of the enzyme, which will subsequently lead to an accumulation of Ca2+ in the cell. Supraphysiological Ca2+ levels will then block the motion apparatus of the sperm cell, lowering the cAMP concentration and restricting the ATP supply with a concomitant fall in its motility [56][70]. High intracellular Ca2+ concentrations may also decrease the level of tyrosine phosphorylation events that are absolutely required for the maintenance of acrosome reaction and capacitation [70]. In addition, QUE exhibited significant inhibitory effects on the hyaluronidase activity and sperm penetration ability in non-capacitated, capacitated and acrosome-reacted cynomolgus monkey sperm in a dose-dependent manner [71].
As such, conflicting biological effects of QUE should be carefully considered before the biomolecule is supplemented to extended spermatozoa. Furthermore, the toxicity of QUE needs to be monitored closely, since its “double-edged sword” behavior is dependent on its dose, species studied in the experiment as well as experimental settings.
Lycopene (ψ,ψ-Carotene) (LYC) is a natural carotenoid, high concentrations of which may be found in ripe tomatoes, watermelons, apricots, pink grapefruits, or papaya [72]. Interestingly enough, the bioavailability of LYC from fresh foodstuffs is relatively low, however this obstacle may be trespassed by heat, processing or co-delivery of fruits and vegetables with foods that contain fat [73].
Although used as a food colorant for decades, LYC has only recently emerged as a subject of scientific interest with respect to its biological activity. LYC is a very powerful antioxidant, which has been shown to quench singlet oxygen twice as effectively as β-carotene and ten times faster when compared to α-tocopherol [72][73].
Numerous reports have shown that this red pigment exhibits protective effects against malignant, cardiovascular, neurodegenerative and eye diseases [72]. It has been reported that LYC accumulates in lipid-rich seminal prostasomes, providing them protection against degradation [74], which is why the molecule is particularly effective in the prevention of prostate cancer [75]. Furthermore, it has been revealed that LYC concentrations in the testes are higher in comparison to other organs indicating that the molecule is likely to play an important physiological role in the process of spermatogenesis [76]. This hypothesis was further fortified by Palan et al. [77] who reported that the concentration of LYC was significantly lower in the seminal plasma collected from infertile men. Additional research [78][79][80] has revealed that the amount of LYC in the seminal plasma increases with oral supplementation of the compound. Summarizing the above-mentioned information, it may be strongly assumed that the intake of LYC will offer a higher level of protection against testicular oxidative stress, and hence possible male reproductive dysfunction.
Although the exact mechanism of action by which LYC exerts its biological activity is still poorly understood, recent studies provide evidence that LYC may be useful in the prevention or management of male infertility (Figure 3).
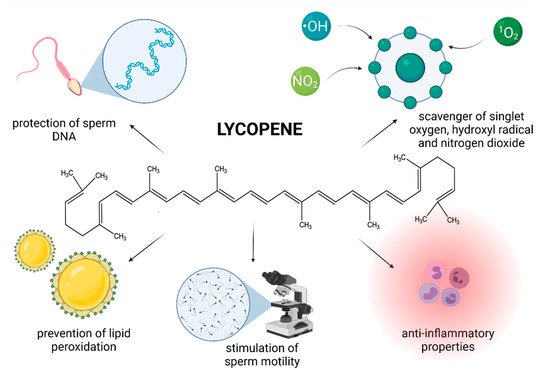
Figure 3. Most frequently reported beneficial effects of lycopene on spermatogenesis, sperm structural integrity and functional activity.
Williams et al. [79] studied the effects of a 12-week supplementation with 14 mg/day of lactolycopene (equivalent to the consumption of 2 kg of cooked tomatoes or 2 tablespoons of concentrated tomato puree) on standard sperm quality parameters of healthy volunteers with no previous fertility issues. Whilst the sperm concentration or DNA integrity were not affected, a significantly improved proportion of fast-progressive sperm as well as a higher amount of morphologically intact male gametes were observed at the end of the experiment, which may be eventually welcomed by infertile men. Indeed, a 12-week LYC supplementation resulted in a significant improvement of the sperm count and concentration in infertile men, however no changes were observed in the sperm morphology [81]. In Mohanty’s et al. study [82], 50 oligoasthenozoospermic males consuming 8 mg LYC over an extended period showed a significant increase in the sperm count and concentration, while according to Gupta and Kumar [83], 30 idiopathic infertile men presented with a 53% increase in sperm concentration following consumption of 2 mg/day LYC for 3 months.
In their study, Yamamoto et al. [80] revealed that infertile patients who consumed tomato juice (containing 30 mg LYC) for 12 weeks, presented with a significantly decreased the concentration of seminal leukocytes suggesting a lower risk for inflammation and sperm damage because of leukocytospermia. Furthermore, the motility rates were significantly higher in the experimental group in comparison with the control indicating that commercially available food, such as tomato juice, might be beneficial to counteract male infertility.
Several clinical trials also focused on the antioxidant properties of LYC in the context of oxidative stress-compromised male reproductive function. Filipcikova et al. [84] observed a significant decrease of MDA production in the seminal plasma of infertile men consuming 10 mg of highly purified LYC from tomato puree and emphasized on the efficacy of LYC in stabilizing the levels of polyunsaturated fatty acids in the sperm membranes. Taş et al. [85] speculated that this LPO-preventing potential lies in the ability of LYC to become entrapped in the hydrophobic core of membranous constituents in spermatozoa. Moreover, LPO was reportedly reduced by consuming tomato juice fortified with vitamin C for 2 weeks [86], where the authors putatively speculated that vitamin C might have acted in a synergy with LYC present in the tomato juice. Interestingly, although a decrease of MDA and a concomitant increase in the GPx activity were observed by Nouri et al. [81], these changes were not statistically significant. Nevertheless, the authors conclude that LYC supplementation may play a role in the improvement in the established functional criteria of fertility, by stabilizing the oxidative balance in semen which may be subsequently translated into improved sperm parameters.
Different mechanisms of action of LYC have been proposed, however the most prominent seems to be mediated via its ability to prevent ROS overproduction. LYC is known to be a highly efficient scavenger of singlet oxygen, hydroxyl radical and nitrogen dioxide [87][88]. As such, the compound may offer protection against oxidation of biomacromolecules crucial for a proper reproductive function [79][81][85]. Moreover, since LYC is lipophilic and frequently found in cell membranes [72][73] it is likely to be present in sufficient amounts to protect critical sperm structures against oxidative insults. Other mechanisms that have been suggested include that of indirectly increasing the amount and/or activity of antioxidant enzymes [76][88] and decreasing the transcription of proinflammatory factors [89][90].
With respect to animal studies, while Mangiagalli et al. [91] reported that administration of 0.1 or 0.5 g/L LYC had no significant impact on the motility rate or forward progressive motility of spermatozoa collected from healthy rabbits, an array of toxicological studies emphasizes on a protective effect that LYC exerts in the male reproductive system compromised by irradiation [92] or drugs [93][94].
According to Dobrzyńska and Gajowik [92] irradiated mice that were exposed to lycopene (0.15 and 0.30 mg/kg) presented with an increased sperm count and a lower percentage of abnormal spermatozoa and/or spermatozoa with a fragmented DNA molecule, suggesting that LYC can ameliorate the harmful effects of irradiation on male gametes.
Türk et al. [93] indicated that LYC administration in rats treated with cyclosporine A (CsA) significantly increased the sperm concentration, motility, and decreased ROS generation in comparison to the CsA-treated control, confirming the role of LYC as a potential protective agent against structural and functional damage as a result of ROS-inflicted testicular damage. A significant antioxidant activity of LYC was furthermore reported by Ateşşahin et al. [94] according to who the presence of LYC significantly improved the semen quality and testicular antioxidant capacity of rats treated with cisplatin. According to Aly et al. [95], LYC supplemented before lipopolysaccharide treatment attenuated the mitochondrial damage in male germ cells. This effect may be attributed to the lipophilic nature of the LYC, which enables the biomolecule to accumulate in the membranous structures and lipoproteins, and subsequently stabilize the mitochondrial metabolism [76]. Protective effects of LYC were accompanied by a decrease of MDA and H2O2 generation, suggesting a ROS-trapping ability of this carotene. Moreover, LYC treatment prevented the testicular decrease of SOD, CAT, GPx and glutathione reductase (GR) activities, normalized GSH and vitamin C concentrations which subsequently contributed to the ROS-scavenging ability of the reproductive system. A stabilization of the testicular oxidative profile alongside an improved activity of SOD, CAT, GPx and GSH followed by a decrease of H2O2 production and MDA synthesis in male reproductive cells and tissues following exposure to bisphenol A [96], cyclosporine A [93] and cisplatin [97] were reported as well.
At the same time, current evidence points out to the potential of LYC as a suitable antioxidant supplement to preservation media for fowl [98], ram [99] and bovine semen exposed to low temperatures [100][101]. All studies agree that LYC supplementation prevented a decline in the sperm motility and prevented the occurrence of sperm abnormalities, acrosome damage or dead sperm, most likely as a result of specific protective effects of this molecule against cell damage through its ROS-quenching abilities and prevention of LPO.
In contrast, Zini et al. [102] observed that pre-incubating human sperm with LYC did not reverse the loss of motility caused by subsequent addition of H2O2 to the culture. Failure of LYC to stabilize sperm motility in this case could be attributed to the presence of high concentrations of H2O2, a well-known lipid soluble ROS which may be able to quickly escape the ROS-trapping abilities of LYC and subsequently curb the sperm activity through numerous oxidative mechanisms. Nevertheless, sperm preincubation with LYC caused a significantly lower DNA damage which may be useful particularly in cases where ICSI is applied, which does depend primarily on the sperm DNA stability rather than its motility [103]. Furthermore Rosato et al. [104] showed that semen extenders containing LYC provided a higher protection to the DNA molecule when turkey semen samples were refrigerated or cryopreserved. Taken together, these studies imply that LYC is able to prevent DNA damage in spermatozoa, therefore increasing the chances of successful fertilization of the oocyte and embryogenesis. This hypothesis is in agreement with previous reports on mammalian germ cells in which the extent of damage to the sperm DNA was assessed using the Comet assay, acridine orange test, the sperm chromatin structure assay or the quantification of 8-OHdG, a direct marker of oxidative damage to DNA [100][101][102][104].
Numerous earlier studies have reported a significant decrease in LPO following LYC supplementation [88][98][101][104]. Nevertheless, Zribi et al. [105] indicated that although oxidative stress was linked to DNA damage, no correlations were recorded between ROS production and MDA amounts. Moreover, several authors have suggested that although LYC is an antioxidant, it failed to reduce the extent of LPO in bovine [106] and ram [107] sperm following cryopreservation. Due to contradictory data on the potential of LYC in preventing peroxidative damage to lipids, it may be hypothesized that LYC may not play a leading role in counteracting LPO during sperm cryopreservation in comparison to other antioxidant supplements traditionally added to the semen extenders [101].
Summarizing the collected data, two hypotheses may emerge to explain positive effects of LYC on either improving or restoring male fertility. One suggests that LYC is a lipophilic substance which easily passes through the cell membranes and quickly enters the cell where it stabilizes and fortifies the inherent antioxidant network. Another one assumes that LYC binds to the membranous structures where it plays an important role in the protection of lipoproteins against oxidative insults. It is plausible to assume that the provitamin A activity of β-carotene has a direct effective role in this protective mechanism of action [108].
Catechins are polyphenolic flavonol compounds which are regarded to be the key bioactive components of green tea. Furthermore, these may be found in black grapes, strawberries, and apricots [109]. Green tea catechins are by and large represented by four biomolecules, specifically epicatechin (EC), epigallocatechin (EGC), epicatechin-3-gallate (ECG) and epigallocatechin-3-gallate (EGCG) [110]. Catechins have been reported to possess a wide spectrum of biological activities and may be useful in the prevention of cardiovascular diseases, cancer, osteoarthritis, and Parkinson’s disease [109][111]. Furthermore, the biomolecules are able to modulate the metabolism of carbohydrates and lipids, thus acting in the prevention of diabetes mellitus Type 2 and liver diseases [111].
Catechin polyphenols are known to possess highly efficient ROS-scavenging activity which has been estimated to be 20 times higher in comparison to vitamin C [112]. These biomolecules are effective chelators of transition metals including cadmium and chromium. Furthermore, the ability of catechins to reduce iron and copper enables them to act in the prevention of the Fenton reaction and a subsequent production of the highly reactive hydroxyl radical [73][113].
In addition, catechins are reported to modulate the levels of monoamines, which control sexual and reproductive behavior. According to Rai et al. [114], 50 mg/kg (+)-catechin was found to enhance sexual behavior in rats, while being safe on the histology of testes, sperm count, motility, and morphology parameters.
Animal models have been used in several in vivo studies to assess the impact of catechins in improving sperm quality, which may provide valuable information for their future potential in the management of male infertility.
According to Ding et al. [115] EGCG administration (50 mg/kg) to mice subjected to intermittent irradiation provided protection against short-term germ cell loss and mitigated radiation-inflicted testicular OS. Furthermore, it was proposed that the biomolecule stimulates and protects spermatogenic recovery and prevents germ cells from radiation-induced apoptosis, leading to a higher sperm production, motility, and a decreased frequency of oxidative insults to the process of spermatogenesis. Awoniyi et al. [116] reported that supplementation of green tea extracts rich in catechins significantly improved the sperm count and motion characteristics of spermatozoa collected from rats treated with TBHP. Furthermore, the authors observed that catechin administration resulted in an improved oxidative profile, stabilization of endogenous antioxidants and prevention of an excessive production of lipid peroxides in epididymal sperm.
In the meantime, Zanchi et al. [117] evaluated the potential of green tea infusion (250 mg/kg) in alleviating cyclophosphamide-induced testicular toxicity in mice. In this study, green tea catechins prevented excessive lipid peroxidation, protein carbonylation, DNA fragmentation, while at the same time, a restored glutathione peroxidase and S transferase activity was observed in mice testes. This stabilization of the oxidative balance was translated into an increased sperm concentration in the epididymis. Green tea catechins were also reported to provide protection against testicular damage induced by doxorubicin. Mice administered with 200 mg/kg green tea presented with a significantly higher sperm density and motility, alongside an improved histology of the testicular tissue [118]. Green tea polyphenols also showed to be effective in the prevention of reproductive failure caused by hyperthermia on semen parameters. Rats subjected to scrotal heat stress which received 500 or 750 mg/kg green tea extracts, presented with a significantly restored sperm concentration, motility, and membrane integrity [119].
Recently, a variety of studies has emerged indicating a potential role of catechin supplementation to sperm storage and/or cryopreservation media. Furthermore, it has been reported that catechins may confer in vitro protection against oxidative insults to male gametes (Figure 4).
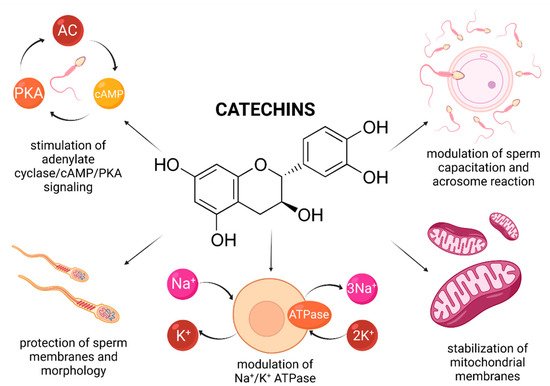
Figure 4. Most frequently reported beneficial effects of catechins on spermatogenesis, sperm structural integrity and functional activity.
In vitro animal studies revealed that catechin concentrations oscillating between 25–100 μmol/L led to a higher motility and membrane integrity preservation of extended and/or cryopreserved bull [120][121], canine [122], caprine [123][124] and boar spermatozoa [125]. This may be explained by a high ability of catechins to be incorporated into the plasma membrane, and thus avoid possible fluctuations in ion concentrations and alterations of the Na+/K+ ATPase, which may have lethal consequences to the sperm survival [126]. Consequently, the stabilization of membrane fluidity resulted in a sperm cell that was better equipped to withstand a variety of physical and environmental assaults [123].
In case of human spermatozoa, it was revealed that lower doses of EGCG (ranging from 2 to 20 μmol/L) affected the estrogen receptor of male gametes, and subsequently increased the cholesterol efflux and tyrosine phosphorylation which manifested itself in an increased sperm motility, viability, and phosphorylation of proteins affecting cell survival (Bcl2, Akt, and Src) [127]. Furthermore, administration of 2 and 20 μmol/L EGCG to the semen extender stimulated the adenylate cyclase/cAMP/PKA signaling, which, alongside protein phosphorylation, plays key roles in the process of sperm capacitation and acrosome reaction [128]. A more detailed molecular analysis revealed that the presence EGCG was associated with a reduction of triglycerides, induction of lipase and G6PDH activity, indicating an increased energy expenditure of the sperm cell. These findings were later confirmed by Spinaci et al. [129] who observed that EGCG treatment increased the number of porcine spermatozoa able to actively bind to the zona pellucida of the oocyte. As such, it may be concluded that catechins may be capable of exerting some impact on the sperm cell, resulting in a modulation of capacitation and acrosome reaction, even under ex vivo conditions.
Enrichment of semen extenders with tea extracts rich in catechins has been reported to lead to an increase in the overall sperm viability and mitochondrial activity [121][122][123]. A possible explanation for the beneficial effects of catechins on the sperm mitochondrial metabolism was offered by Uekusa et al. [130] who took advantage of nuclear magnetic resonance spectroscopy to reveal a high affinity of catechins for the sperm mitochondrial membranous structures. A proper stabilization of the mitochondrial integrity, alongside prevention of ROS overgeneration in the presence of catechins may ensure a more effective function of the sperm mitochondrial compartment. Furthermore, Nagata et al. [131] reported that a synergism exists between catechin and glutathione peroxidase, that may contribute to a more effective stabilization of the intracellular antioxidant system of the male gamete [121].
Despite the beneficial effects of catechins on male fertility as mentioned above, their possible negative effects on male reproductive organs and cells have been reported by other authors. Relatively high doses of catechins have been shown to cause inhibition of spermatogenesis alongside alterations to the morphology and activity of spermatozoa [132].
According to Jamalan et al. [133], high concentrations of catechins (25–1000 μmol/L) did not exhibit any significant protective effect on human spermatozoa exposed to cadmium, aluminum, or lead. What is more, Moretti et al. [63] observed that 200 μmol/L and 400 μmol/L EPI had a compromising effect on human sperm motion behavior, overall viability, or the extent of LPO when male gametes were subjected to TBHP. A similar observation was reported by Silva et al. [124] who observed that the treatments with (+)-catechin or EGCG, at higher concentrations (50 to 100 µmol/L), had an inhibitory effect on goat sperm kinematics.
A possible explanation for this phenomenon may lie in the ability of high doses of catechins to inhibit F0F1-ATPase and cyclooxygenase (COX) which contribute to ATP synthesis. Furthermore, COX modulates the production of prostaglandins, which affect sperm motility [31][134]. Finally, it has been postulated that elevated concentrations of catechins may exhibit antiestrogenic properties [127]. In this sense, catechins may be defined as natural dose-dependent motility inhibitors. As such, it must be emphasized that the dosage may be the single most important factor deciding whether catechins will exhibit beneficial or detrimental effects on the sperm function.
Another aspect that must be taken into consideration is an increasing evidence on “double-edged sword” properties of catechins, as these may be converted to pro-oxidant derivatives [135]. Catechins are relatively unstable and can contribute to oxidative tension by undergoing autooxidation. According to Sang et al. [136] the stability of catechins directly depends on their concentration, incubation temperature, pH of the semen extender or the presence of oxygen. As such, all these variables must be considered before catechins may be used as supplements for sperm processing and preservation.