Nature has been a source of inspiration for the development of new pharmaceutically active agents. Polyphenols, including gallotannins, are widely studied as they protect cells against oxidative damage and pathogen attack. A series of new unnatural gallotannins (GTs), derived from D-lyxose, D-ribose, D-rhamnose, D-mannose, and D-fructose have been designed and synthesized i order to study the protective and antimicrobial effects of synthetic polyphenols that are structurally related to plant-derived products. Apart from spectral analysis, their antioxidant activity was evaluated. Structurally different GTs were screened in vitro for their antimicrobial properties against a spectrum of staphylococci, enterococci, and mycobacteria. Furthermore, the antibiofilm activity of GTs against S. aureus, and their ability to inhibit sortase A were inspected. Experimental data suggest that synthetic GTs could be considered as promising candidates for pharmacological, biomedical, and food industry applications.
1. Introduction
Tannins are a large sub-class of polyphenolic compounds ubiquitously present in plants. They are found in a variety of species, playing roles in the plant’s natural defence system against environmental stressors and microbial infections
[1][2][3]. Natural tannins are widely studied for their prophylactic and therapeutic potential
[4][5]. Gallotannins (GTs) from various species have been extensively studied as they exhibit multiple biological activities ranging from antioxidant, radical scavenging, antimicrobial, anti-inflammatory, and immune-modulatory to anticancer effects
[2][6][7][8][9]. Moreover, numerous plant polyphenols have exhibited strong antibacterial and antibiofilm activity against staphylococci
[10][11]. Among the staphylococci,
Staphylococcus aureus is of most clinical concern. Undesirable bacterial
S. aureus biofilm layers are formed on indwelling medical devices or food processing contact-surfaces, resulting in microbial communities more resistant to the traditional disinfectants
[12][13][14].
The molecular structure of GTs is generally composed of a central carbohydrate core esterified with gallic acid (GA). Structurally related polyphenolic compounds, e.g., 1,2,3,6-tetra-
O-galloyl-
D-galactose
[15], 1-
O-galloyl-
L-rhamnose
[16], 7-
O-galloyl-D-sedoheptulose
[17], 1,2,3,4,6-penta-
O-galloyl-
D-glucose
[7][18][19], 2,3-di-
O-galloyl-D-glucitol, or 2,3,6-tri-
O-galloyl-
D-glucitol
[20], exert interesting biological activities. Among the vast number of bioactive polyphenols, 1,2,3,4,6-penta-
O-galloyl-
D-glucose (PGG), has been the most widely studied. A number of in vitro and in vivo studies have shown that PGG exhibits diverse pharmacological effects
[7][19][21]. Interesting anti-staphylococcal activity was observed for PGG isolated from the Thai mango (
Mangifera indica L.). The antibacterial investigation of a crude GT extract on
S. aureus revealed that PGG was the most effective component
[22]. The effect of the extract was also synergistic with penicillin G. Damaging effects on the cell membrane, leading to an alteration in cell morphology and interference with bacterial division, were suggested as a possible inhibitory mechanism. Moreover, the PGG exhibited a remarkable anti-biofilm activity. It was observed that PGG noticeably inhibited the initial phase of biofilm formation of
S. aureus [23]. Natural PGG, isolated from
Paeonia suffruticosa, was evaluated for its antifungal activity in vitro, against
Candida glabrata. According to the MIC values, PGG was 10-fold more effective than the standard antifungal drug fluconazole. It was demonstrated that the antifungal activity of PGG is mediated by local ruptures in the cell wall, but that these did not affect plasmatic membrane, nucleus or mitochondria
[24].
Galloylated branched-chain sugars, are a rare class of naturally occurring GTs. A typical example is 2′,5-di-
O-galloyl-2-
C-(hydroxymethyl)-
D-ribose (hamamelitannin) which is an active component of various plant extracts
[25][26][27]. The antioxidant and radical scavenging effect of hamamelitannin have been studied, and the respective molecular mechanisms were described in detail
[28][29][30]. Natural hamamelitannin has also been reported as efficient antiviral, antibacterial, antibiofilm, anti-inflammatory, and anticancer agent
[31][32][33][34].
The biological effects of galloylated branched-chain sugars have not been investigated in detail due to the difficulties with isolation of the pure compounds from plants. Solution toward this end is the synthesis of naturally identical compounds or their analogues. As a part of ongoing studies on biologically important sugars as potential drug candidates, new galloyl-derivatives of 2-C-hydroxymethyl-branched sugars derived from D-lyxose, D-ribose, D-mannose, L-rhamnose, D-fructose were synthesized and compared their biological activities with the unnatural GTs (methyl tetra-O-galloyl-α-D-glucoside, methyl tetra-O-galloyl-α-D-mannoside, methyl tri-O-galloyl-α-L-rhamnoside [35]), penta-O-galloyl-D-glucose, and gallic acid. The effects of GTs on environmental and human pathogens were examined in various experimental systems. Structurally different GTs were screened in vitro for their antimicrobial properties against a spectrum of staphylococci, enterococci, and mycobacteria. Their ability to eradicate pre-formed bacterial biofilms of S. aureus, and quorum sensing (QS) inhibition in Chromobacterium violaceum was also examined.
2. Results and Discussion on New Unnatural Gallotannins
2.1. Chemistry
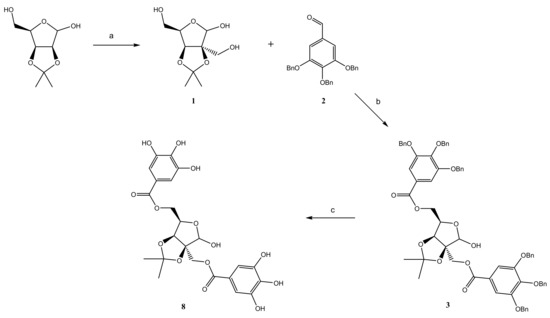
The presence of multiple galloyl units in the GT molecules make them powerful antioxidants as well as effective antimicrobial agents. The strong contribution of the galloyl groups to these properties has been demonstrated several times, but it has also been observed that the carbohydrate moiety also plays an important role as well
[35]. Carbohydrates are characterized by structural diversity and a multiplicity of nearly equivalent hydroxyl groups. As the 2-
C-(hydroxymethyl)-branched-chain aldoses have several different hydroxyl groups, it was important to choose a suitable approach for the synthesis of their galloyl-esters. For that reason, 2,3-
O-isopropylidene derivatives of the 2-
C-(hydroxymethyl)-branched aldoses were chosen as suitable compounds. During the synthesis, all phenolic hydroxyl groups of GA were protected by benzylation to avoid intra- and inter-molecular stacking interactions between galloyl units. Hence, the appropriately protected 2-
C-(hydroxymethyl)-branched aldose was esterified with benzylated GA (
2) in the presence of DMAP as a catalyst and DCC as a coupling reagent, in order to obtain 2,3,4-tri-
O-benzyl-galloylated 2-
C-(hydroxymethyl)-branched aldoses (derived from D-Lyx, D-Rib, L-Rham, D-Man
3–
6) and the 2,3,4-tri-
O-benzyl-galloyl derivative of
D-fructose (
7) in very good yields. Subsequent debenzylation of compounds
3–
7 led to the expected galloylated branched-chain aldoses (derived from D-Lyx, D-Rib, L-Rham, D-Man
8–
11) and galloylated
D-fructose (
12) in excellent yields. A representative procedure for the synthesis of the 2′,5-di-
O-galloyl-2-
C-(hydroxymethyl)-2,3-
O-isopropylidene-
D-lyxofuranose (
8, G
2Lyx) is depicted in
Scheme 1. The structures of the new derivatives were determined on the basis of NMR spectroscopy and other analytical methods.
Scheme 1. Synthesis of 2′,5-di-O-galloyl-2-C-(hydroxymethyl)-2,3-O-isopropylidene-D-lyxofuranose (8).
2.2. Antioxidant Activity
The antioxidant activity of compounds is related to their redox properties, which allow them to scavenge free radicals by acting as hydrogen donors or reducing agents. A series of structurally different GTs (
Figure 1), were screened for their free-radical-scavenging effect and reducing ability using the DPPH and FRAP assays. It is known that GA is a strong antioxidant due to the presence of three hydroxyl groups on the aromatic ring
[36]. Thus, the high antioxidant efficiency of these compounds could be attributed to the presence of multiple galloyl groups in their structures. The experimental results indicated that the studied GTs exhibited notably different and concentration-dependent DPPH radical-scavenging effects (
Table 1). Compounds PGG, G
4Man, G
3Rham, and G
4Glc exhibited the highest radical-scavenging activity (94–98%), whereas the di-galloylated 2-
C-(hydroxymethyl)-branched aldoses (G
2Rib, G
2Lyx, and G
2Rham) manifested a slightly lower effect (85–88%). Moreover, as expected, mono-galloylated derivatives (GMan and GFru) displayed only moderate DPPH radical-scavenging activity (71–73%). Almost equal antioxidant activity was observed for PGG (98%) and G
4Glc (96%), where the latter differs from the former by one less galloyl group. Moreover, comparable radical-scavenging activity was also observed for the compounds G
4Man (95%), G
3Rham (94%) and G
4Glc (96%), which have very similar molecular structures.
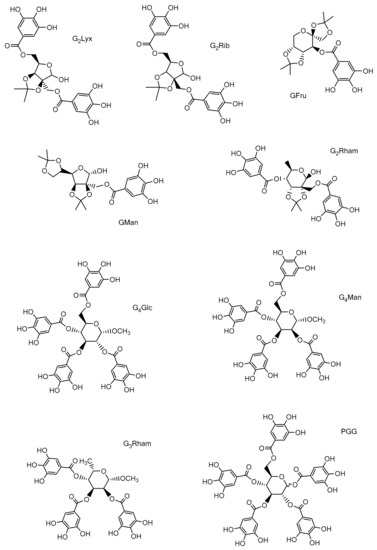
Figure 1. Studied compounds: 2′,5-di-O-galloyl-2-C-(hydroxymethyl)-2,3-O-isopropylidene-D-lyxose (G2Lyx); 2′,5-di-O-galloyl-2-C-(hydroxymethyl)-2,3-O-isopropylidene-D-ribose (G2Rib); 2′,4-di-O-galloyl-2-C-(hydroxymethyl)-2,3-O-isopropylidene-L-rhamnose (G2Rham); 2′-O-galloyl-2-C-(hydroxymethyl)-2,3:5,6-di-O-isopropylidene-D-mannose (GMan); 3-O-galloyl-1,2:4,5-di-O-isopropylidene-D-fructose (GFru); methyl 2,3,4,6-tetra-O-galloyl-α-D-glucoside (G4Glc); methyl 2,3,4,6-tetra-O-galloyl-α-D-mannoside (G4Man); methyl 2,3,4-tri-O-galloyl-α-L-rhamnoside (G3Rham); 1,2,3,4,6-penta-O-galloyl-D-glucose (PGG).
Table 1. Antioxidant activity. DPPH radical-scavenging assay and reducing power of 2′,5-di-O-galloyl-2-C-(hydroxymethyl)-2,3-O-isopropylidene-D-lyxose (G2Lyx); 2′,5-di-O-galloyl-2-C-(hydroxymethyl)-2,3-O-isopropylidene-D-ribose (G2Rib); 2′,4-di-O-galloyl-2-C-(hydroxymethyl)-2,3-O-isopropylidene-L-rhamnose (G2Rham); 2′-O-galloyl-2-C-(hydroxymethyl)-2,3:5,6-di-O-isopropylidene-D-mannose (GMan); 3-O-galloyl-1,2:4,5-di-O-isopropylidene-D-fructose (GFru); methyl 2,3,4,6-tetra-O-galloyl-α-D-glucoside (G4Glc); methyl 2,3,4,6-tetra-O-galloyl-α-D-mannoside (G4Man); methyl 2,3,4-tri-O-galloyl-α-L-rhamnoside (G3Rham); 1,2,3,4,6-penta-O-galloyl-D-glucose (PGG).
| Comp. |
Concentration
(mM) |
DPPH Scavenging Activity (%) |
Reducing Power
(Absorbance) |
| G2Lyx |
0.1 |
49.97 ± 1.14 |
0.675 ± 0.041 |
| 0.25 |
63.98 ± 1.05 |
0.886 ± 0.015 |
| 0.5 |
75.35 ± 1.61 |
1.263 ± 0.053 |
| 1 |
87.36 ± 1.35 |
1.699 ± 0.082 |
| G2Rib |
0.1 |
52.67 ± 1.27 * |
0.698 ± 0.028 |
| 0.25 |
67.55 ± 1.08 * |
0.979 ± 0.015 |
| 0.5 |
78.39 ± 1.11 |
1.185 ± 0.031 |
| 1 |
87.95 ± 1.39 |
1.761 ± 0.102 |
| G2Rham |
0.1 |
49.68 ± 2.13 |
0.622 ± 0.036 |
| 0.25 |
67.04 ± 1.72 |
0.904 ± 0.045 |
| 0.5 |
79.63 ± 1.21 |
1.325 ± 0.018 |
| 1 |
85.18 ± 1.03 |
1.711 ± 0.072 |
| GMan |
0.1 |
33.57 ± 1.66 *** |
0.534 ± 0.081 * |
| 0.25 |
48.19 ± 2.09 ** |
0.871 ± 0.016 * |
| 0.5 |
68.42 ± 1.74 * |
1.054 ± 0.037 * |
| 1 |
71.28 ± 1.16 ** |
1.128 ± 0.057 ** |
| GFru |
0.1 |
27.67± 1.25 *** |
0.485 ± 0.044 * |
| 0.25 |
39.29 ± 1.08 *** |
0.621 ± 0.073 ** |
| 0.5 |
62.18 ± 1.13 *** |
0.823 ± 0.051 ** |
| 1 |
73.36± 2.19 *** |
1.105 ± 0.069 ** |
| G4Glc |
0.1 |
69.72 ± 1.59 *** |
0.826 ± 0.017 * |
| 0.25 |
75.35 ± 1.98 ** |
1.297 ± 0.044 ** |
| 0.5 |
84.49 ± 2.27 * |
1.999 ± 0.081 ** |
| 1 |
95.96 ± 1.77 ** |
2.267 ± 0.086 ** |
| G4Man |
0.1 |
68.19 ± 1.54 *** |
0.779 ± 0.051 |
| 0.25 |
77.02 ± 2.11 ** |
1.307 ± 0.079 *** |
| 0.5 |
83.76 ± 1.99 * |
1.878 ± 0.068 ** |
| 1 |
95.01 ± 1.63 ** |
2.238 ± 0.039 ** |
| G3Rham |
0.1 |
71.38 ± 1.74 *** |
0.801 ± 0.073 * |
| 0.25 |
78.14 ± 1.49 ** |
1.464 ± 0.057 ** |
| 0.5 |
86.63 ± 2.08 * |
1.935 ± 0.108 *** |
| 1 |
94.13 ± 1.27 ** |
2.197 ± 0.096 ** |
| PGG |
0.1 |
75.32 ± 2.01 *** |
0.918 ± 0.025 * |
| 0.25 |
80.27 ± 1.14 *** |
1.506 ± 0.037 ** |
| 0.5 |
92.35 ± 1.58 ** |
2.104 ± 0.093 *** |
| 1 |
97.88 ± 1.22 ** |
2.307 ± 0.034 ** |
| GA |
0.1 |
47.56 ± 1.45 |
0.688 ± 0.015 |
| 0.25 |
63.03 ± 2.06 |
1.002 ± 0.071 |
| 0.5 |
76.89 ± 1.73 |
1.289 ± 0.059 |
| 1 |
88.14 ± 1.16 |
1.736 ± 0.084 |
The variation in the radical-scavenging effect amongst the tested GTs was probably due to the different stability of the resulting oxygen-centred radical formed in these compounds. The studied GTs exhibited varying ability to reduce Fe3+/ferricyanide complex to Fe2+/ferrocyanide, as seen in Table 1. Increases of the reducing power were correlated with the concentration of the tested compound, as well as with the number and substitution pattern of the galloyl groups attached to the carbohydrate core. The compounds PGG and unnatural GTs (G4Glc, G4Man, and G3Rham) exhibited remarkable potency for donating electrons to reactive free radicals, transforming them into more stable species. However, the presence of isopropylidene groups in galloylated 2-C-(hydroxymethyl)-branched aldoses (G2Rib, G2Lyx, and G2Rham) resulted in a noticeable decrease of their reducing power.
The experimental data thus revealed that the majority of the studied compounds exhibit an excellent radical-scavenging and reducing activity. The results (
Table 1) demonstrated that outcomes from the FRAP assay are in good agreement with those of the DPPH assay, and that the overall antioxidant activity of each tested GT can be regarded as the sum of contributions of all the structural features in the molecule, depending on the number of galloyl groups and type of carbohydrate core, and the individual galloyl groups’ contributions varying with their position and spatial arrangement. These findings are consistent with other reports on antioxidant potential of phenolic acids
[37].
2.3. Antibacterial and Antimycobacterial Activity
The antibacterial activity of the unnatural GTs was tested against S. aureus ATCC 29213, three MRSA isolates, E. faecalis ATCC 29212, and three VRE isolates. For screening of antimycobacterial effects a virulent isolate of M. tuberculosis and two non-tuberculous mycobacteria were used. The summary of results from experiments is presented in Table 2.
Table 2. Antimicrobial activities of investigated compounds against Gram-positive bacteria and mycobacteria expressed as a minimum inhibitory concentration (MIC [µg/mL]/[mM]) compared to gallic acid (GA), ampicillin (AMP) and isoniazid (INH).
| Comp. |
MIC ([µg/mL]/[mM]) |
| SA |
MRSA-1 |
MRSA-2 |
MRSA-3 |
EF |
VRE-1 |
VRE-2 |
VRE-3 |
MT |
MK |
MS |
| G4Glc |
256/
318 |
256/
318 |
64/
79.7 |
64/
79.7 |
32/
39.8 |
64/
79.7 |
256/
318 |
256/
318 |
>128/
>159 |
256
318 |
256/
318 |
| G4Man |
64/
79.7 |
256/
318 |
64/
79.7 |
64/
79.7 |
16/
19.9 |
32/
39.8 |
256/
318 |
256/
318 |
>128/
>159 |
128/
159 |
256/
318 |
| G3Rham |
128/
201 |
256/
403 |
128/
201 |
128/
201 |
32/
50.4 |
128/
201 |
256/
403 |
256/
403 |
>128/
>201 |
128/
201 |
256/
403 |
| G2Lyx |
>256/
>504 |
>256/
>504 |
>256/
>504 |
>256/
>504 |
>256/
>504 |
>256/
>504 |
>256/
>504 |
>256/
>504 |
>128/
>252 |
>256/
504 |
>256/
504 |
| G2Rham |
256/
456 |
256/
456 |
256/
456 |
256/
456 |
>256/
>456 |
>256/
>456 |
>256/
>456 |
>256/
>456 |
>256/
>456 |
>256/
>456 |
>256/
>456 |
| G2Rib |
128/
244 |
256/
488.2 |
256/
488 |
128/
244 |
>256/
>488 |
>256/
>488 |
>256/
>488 |
>256/
>488 |
>128/
>244 |
>256/
>488 |
>256/
>488 |
| GMan |
128/
289 |
256/
578 |
256/
578 |
128/
289 |
>256/
>578 |
>256/
>578 |
>256/
>578 |
>256/
>578 |
>128/
>289 |
>256/
>578 |
>256/
>578 |
| GFru |
128/
310 |
128/
310 |
256/
620 |
128/
310 |
>256/
>620 |
>256/
>620 |
>256/
>620 |
>256/
>620 |
>128/
>310 |
>256/
>620 |
>256/
>620 |
| PGG |
32/
34.0 |
128/
136 |
32/
34.0 |
16/
17.0 |
16/
17.0 |
64/
68.0 |
128/
136 |
256/
272 |
>128/
>136 |
256/
272 |
>256/
>272 |
| GA |
256/
1487 |
64/
371 |
64/
371 |
32/
185 |
>256/
>1487 |
>256/
>1487 |
>256/
>1487 |
>256/
>1487 |
>128/
>743 |
256/
1487 |
>256/
>1487 |
| AMP |
2/
5.72 |
16/
45.8 |
>16/
>45.8 |
>16/
>45.8 |
1/
2.86 |
4/
11.5 |
4/
11.5 |
4/
11.5 |
– |
– |
– |
| INH |
– |
– |
– |
– |
– |
– |
– |
– |
8
58.0 |
4
29.1 |
16
117 |
It can be concluded that most of the tested compounds were ineffective against both staphylococci and enterococci (> 128 µg/mL). PGG and unnatural epimeric GTs (G4Man and G4Glc) showed the highest potency (in the range of 16–64 µg/mL) within the tested series of compounds. In addition, the activity of these derivatives against enterococci is insignificant. Thus, the activity was not significantly affected by the presence of the mecA gene (in MRSA clinical isolates) or vanA (VRE clinical isolates). It can be summarized that unnatural G3Rham, galloylated 2-C-(hydroxymethyl)-branched aldoses (G2Rib, G2Lyx, G2Rham, and GMan), and mono-galloyl fructose (GFru) with isopropylidene groups in their structures were less active than PGG, G4Glc, and G4Man (Table 2). The antimycobacterial activity of the tested GTs was also insignificant. The compounds PGG, G4Glc, and G4Man showed only moderate antimycobacterial effect against M. tuberculosis (MIC ≥ 128 µg/mL). The galloylated 2-C-(hydroxymethyl)-branched aldoses (G2Rib, G2Lyx, G2Rham, and GMan), bearing isopropylidene groups did not show any antimycobacterial activity.
Several studies with similar natural compounds can be found in the literature. The antistaphylococcal activity of Thai mango (
M. indica) kernel extract, containing more than 60% PGG and less than 1% gallic acid and methyl gallate, was studied in detail. The antibacterial effect was caused by PGG (MIC = 160 µg/mL) and was comparable with our results
[22]. The group of Chan et al. investigated the antistaphylococcal activity of galloyl-substituted flavonol-rhamnosides extracted from
Calliandra tergemina leaves. Three compounds, kaempferol-3-
O-(2,3,4-tri-
O-galloyl)-α-
L-rhamnopyranoside, quercetin-3-
O-(3,4-di-
O-galloyl)-α-
L-rhamnopyranoside and quercetin-3-
O-(2,3,4-tri-
O-galloyl)-α-
L-rhamnopyranoside, showed only insignificant activity against three MRSA isolates (MIC 256 µg/mL). Compounds bearing only one galloyl group showed no anti-MRSA activity. However, multiple esterification of
L-rhamnose molecule increased the antibacterial effect. Scanning electron microscopy (SEM) studies revealed that these compounds interact with the bacterial membrane
[38]. Derivatives of gallic acid are able to interact with different bacterial cell structures. As mentioned above, one of the mechanisms of action is the interaction with the plasma membrane. This interaction leads to leakage of cell proteins, nucleic acids, or inorganic compounds. Disruption of the cell membrane subsequently causes cell shrinkage and cell lysis, resulting in cell death
[38]. In addition, GTs are also able to chelate metal ions, such as copper and iron, and make them inaccessible to microorganisms. For example,
Terminalia chebula extract, containing significant amount of 1,2,6-tri-
O-galloyl-
D-glucose, demonstrated antibacterial activity against multidrug-resistant uropathogens, which might be due to iron-complexing properties
[39]. PGG itself is able to interact directly with the bacterial cell wall. Zhao et al. studied PGG activity against
Candida glabrata. The compounds were 10 times more active than fluconazole. The antifungal effect was due to the direct interaction of PGG with the fungal cell wall, without any interaction with glucan synthase
[24].
2.4. Antibiofilm Activity
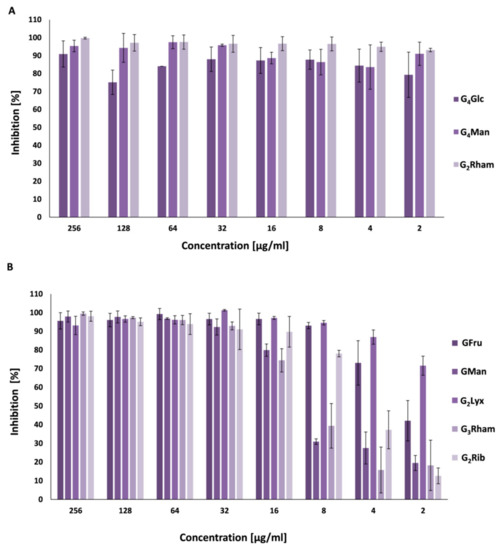
Bacterial biofilms protect the microbial community from external damage and increase the persistence of chronic infections. Selected compounds were examined as inhibitors and disruptors of S. aureus ATCC 29213 biofilms. The results indicate that both groups, PGG analogues (Figure 2A) and galloylated 2-C-(hydroxymethyl)-branched aldoses (Figure 2B), efficiently inhibited S. aureus biofilm formation at concentrations lower than MIC values. For example, the minimum biofilm inhibition concentration (MBIC) of compound G4Glc was 64 times lower than its MIC value, MBIC for G4Man was 32 times lower, and MBIC for G3Rham was 256 times lower than its MIC value. These results identify G3Rham as the most potent inhibitory compound from this series.
Figure 2. Inhibitory activities of compounds against S. aureus ATCC 29213 biofilm formation. Compounds: (A) methyl 2,3,4,6-tetra-O-galloyl-α-D-glucoside (G4Glc); methyl 2,3,4,6-tetra-O-galloyl-α-D-mannoside (G4Man); methyl 2,3,4-tri-O-galloyl-α-L-rhamnoside (G3Rham). (B) 3-O-galloyl-1,2:4,5-di-O-isopropylidene-D-fructose (GFru); 2′-O-galloyl-2-C-(hydroxymethyl)-2,3:5,6-di-O-isopropylidene-D-mannose (GMan); 2′,5-di-O-galloyl-2-C-(hydroxymethyl)-2,3-O-isopropylidene-D-lyxose (G2Lyx); 2′,4-di-O-galloyl-2-C-(hydroxymethyl)-2,3-O-isopropylidene-L-rhamnose (G2Rham); 2′,5-di-O-galloyl-2-C-(hydroxymethyl)-2,3-O-isopropylidene-D-ribose (G2Rib).
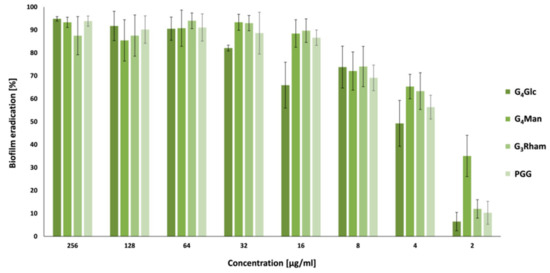
New anti-biofilm compounds that can effectively eradicate bacterial biofilms are desirable to prevent various infections. Eradication activity was tested for PGG and its analogues G4Glc, G4Man and G3Rham. All tested compounds eliminated 80% of the matured biofilm of S. aureus at concentrations lower than the MIC value against planktonic cells. In this case, as well, G3Rham was the most active compound. G3Rham eradicated biofilms of S. aureus at a concentration 16 times lower than its MIC value (Figure 3). The interesting biofilm inhibitory and eradication activity against S. aureus make unnatural GTs excellent biofilm-controlling agents for healthcare and food processing facilities.
Figure 3. Eradication activities of compounds against S. aureus ATCC 29213 biofilm formation. Compounds: methyl 2,3,4,6-tetra-O-galloyl-α-D-glucoside (G4Glc); methyl 2,3,4,6-tetra-O-galloyl-α-D-mannoside (G4Man); methyl 2,3,4-tri-O-galloyl-α-L-rhamnoside (G3Rham) and 1,2,3,4,6-penta-O-galloyl-D-glucose (PGG).
A number of structurally similar natural GTs with remarkable antimicrobial and antibiofilm properties have been studied. It was reported that extracts of
Cytinus possess interesting antimicrobial activity against Gram-positive bacteria (
S. aureus, Staphylococcus epidermidis and
Enterococcus faecium). Characterization of the tannin profile of
Cytinus hypocistis and
Cytinus ruber revealed a significant number of GTs, in particular 1-
O-galloyl-β-
D-glucose and PGG. Three pathogenic species were sensitive to
Cytinus extracts, and a significant inhibition of
S. epidermidis biofilm formation was also observed
[40]. The antimicrobial activity of GT, 1,2,6-tri-
O-galloyl-β-
D-glucopyranose, isolated from
T. chebula, against multidrug-resistant
E. coli was investigated. This tri-
O-galloyl derivative of
D-glucose, was effective against multidrug-resistant uropathogens, and acted synergistically in combination with gentamicin and trimethoprim and additively in combination with amoxicillin, ciprofloxacin and ceftazidine. This substance was able to eradicate the preformed
E. coli biofilms and at the same time act with synergistic antibiofilm activity in combination with gentamicin and trimethoprim
[41]. This compound differs from the test compound G
4Glc only by the number and position of the gallic acid residues in the molecule. It was shown that natural hamamelitannin (which is structurally very similar with test compound G
2Rib) interfaces with QS in
S. aureus, and thereby increases the susceptibility of
S. aureus biofilms to various antibiotics
[34]. Overall, the results demonstrate that unnatural GTs have sufficient inhibitory effect against
S. aureus ATCC 29213 biofilm formation.
2.5. Quorum Sensing Inhibition
Quorum sensing is a communication mechanism that regulates bacterial virulence in pathogens. A number of plant phenolics have been identified as important microbial growth and QS inhibitors
[42]. It was expected that the compounds could work as inhibitors of the QS system, as they interacted with
S. aureus biofilms at concentrations lower than MIC values. This activity was tested on the opportunistic bacterium
C. violaceum. One particular characteristic of this genus is the synthesis of the purple pigment, violacein, which is regulated by QS. Inhibition of this system blocks violacein production and leads to the growth of white or colourless colonies of
C. violaceum. Interestingly, all tested compounds, except for G
2Rham, inhibited violacein production (
Table 3). However, it was not possible to determine whether the observed effect was related to the sugar moiety or to the number of galloyl groups due to the slightly different activities of GTs. Nevertheless, it is important to be aware of the fact that QS active compounds have non-linear effects
[43].
Table 3. Inhibitory activity of compounds to QS system of C. violaceum.
Comp.
(conc. 10 mg/mL) |
Diameter of Inhibition Zone on Agar
[mm] |
| G4Glc |
5.00 ± 0.01 |
| G4Man |
8.50 ± 2.90 |
| G3Rham |
7.00 ± 1.40 |
| G2Rham |
– |
| G2Rib |
8.30 ± 0.62 |
| G2Lyx |
5.00 ± 1.01 |
| GMan |
3.00 ± 0.02 |
| GFru |
6.50 ± 1.31 |
| PGG |
6.00 ± 0.01 |
2.6. Inhibition of Sortase A
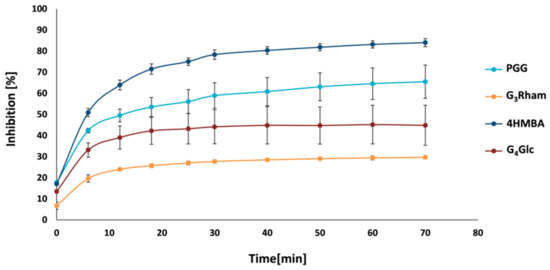
Since SrtA, which anchors surface proteins to the cell wall, plays a critical role in Gram-positive bacterial pathogenesis, it should be possible to target bacterial virulence and treat infections by inhibiting the activity of SrtA. The structurally different compounds PGG, G4Glc and G3Rham were investigated as potential inhibitors of SrtA. The experiments indicated that these compounds are able to block the pathogenic action of SrtA, and the degree of enzyme inhibition is plotted against time in Figure 4. PGG (bearing five galloyl units in its structure) exhibited the most potent inhibitory effect against SrtA among all tested compounds. It inhibited SrtA by 65.5 ± 7.9% after 70 min. The tetra- and tri-O-galloylated compounds G4Glc and G3Rham had approximately half (44.8 ± 9.5%) and quarter inhibitory activity (29.6 ± 0.8%), respectively. These findings indicate that the ability to inhibit SrtA increases with the degree of galloylation. Thus, the number of galloyl groups attached to the carbohydrate core seems to be a critical factor.
Figure 4. Inhibitory activity of compounds against SrtA. Compounds: 1,2,3,4,6-penta-O-galloyl-D-glucose (PGG); methyl 2,3,4-tri-O-galloyl-α-L-rhamnoside (G3Rham) and methyl 2,3,4,6-tetra-O-galloyl-α-D-glucoside (G4Glc); 4-(hydroxymercuri)benzoic acid (4HMBA).
The ability of PGG derivatives to inhibit SrtA has not been described in the literature yet. However, there are many studies examining the effect of other polyphenolic compounds, mainly flavonoids, on bacterial sortases. It was observed that the flavonoid baicalin binds to sortase B in
S. aureus and reduces its virulence
[44]. Other flavonoids, especially kurarinol, isolated from the root of
Sophora flavescens, acted as inhibitors of SrtA as well
[45]. Other sortase inhibitors, which are potentially useful in the treatment of bacterial infections, include isovitexin
[46], astilbin
[47], and rutin
[48].
The findings described above suggest that PGG and its structural analogues G4Glc and G3Rham are able to inhibit the catalytic activity of SrtA in vitro, but have little effect on bacterial growth. The compounds interfered with the adhesion of S. aureus biofilm, and thus hold promise for the development of anti-virulence agents against medical device infections caused by S. aureus. It seems that unnatural GTs may be an important new direction in the field of research into SrtA inhibitors.
3. Future directions
In summary, evaluation of antioxidant, antimicrobial, and antibiofilm activity showed the differences among synthetic GTs, PGG and gallic acid. Unnatural GTs exhibited strong antioxidant and radical-scavenging activity. The compounds inhibited StrA, and it can be stated that the inhibitory effect increased with the increasing number of galloyl groups. On the other hand, the inhibitory effect was also dependent on the type of sugar. It can be hypothesized that the observed antibacterial activity is related to the inhibition of SrtA, although, there are other mechanisms which are also likely to be involved. All investigated unnatural GTs proved to be potent inhibitors and disruptors of S. aureus biofilms that makes these GTs valuable alternatives to currently used antibiofilm agents suitable for application in the biomedical, pharmaceutical, cosmetics, consumer products, and food industries.