1000/1000
Hot
Most Recent

The incidence rate of post-COVID lung fibrosis can be estimated at 2–6% after a moderate illness. According to this estimation, the prevalence of post-COVID lung fibrosis will be from 10 to 30 patients per 10,000 populations, which is 30 times higher than the IPF prevalence.
The outbreak of a novel coronavirus strain, SARS-CoV-2, in December 2019 triggered the ongoing pandemic of the acute respiratory coronavirus-induced disease 2019 (COVID-19, also simply referred to as COVID) [1]. As the new treatments and approaches towards the reduction of disease mortality emerged, new data hinted at a “hidden pandemic” of long-term sequelae of severe COVID [2]. Observational studies indicate that 90% of COVID patients experience COVID sequelae such as respiratory problems, decreased exercise tolerance, and lung tissue damage [3]. These conditions are resolved within 3 months of hospitalization in 50% of the patients [3][4]. However, between 3 and 6 months after hospitalization, the rate of full recovery drops to 35%, and between 6 and 9 months, it goes down to just 15% [5]. The rate of complete recovery from lung damage specifically is 50% during the first 6 months after hospitalization and 75% during a 9-month period. At 9 months following hospitalization, 30% of the patients experience at least some COVID sequelae [5]. Furthermore, the majority of that population has residual lung tissue damage, with nearly a third (or 10% of all patients) exhibiting pronounced fibrotic lung damage; the majority of the patients in the latter group experienced severe respiratory pathology and decreased exercise tolerance. Further, observation did not reveal any trends in the reduction of the severity or frequency of COVID sequelae [5].
Elderly people with severe coronavirus symptoms or dangerous pre-existing health conditions are at the highest risk for post-COVID fibrosis (although the term “fibrosis” applies to irreversible disease, there is no consensus about the type of “fibrotic-like” sequelae in post-COVID patients [6]. Hence, in literature post-COVID fibrosis can be described as either organizing pneumonia [7], or interstitial lung disease [8][9], or just pulmonary fibrosis [10], or fibrotic lung disease [11] in general. Thus, here, by the term “post-COVID fibrosis”, we mean any “fibrotic-like” conditions.) [12]. Fibrosis was clinically confirmed in 56% of the patients who experienced moderate COVID symptoms and in 71% of the patients with severe symptoms, 3 months after they recovered from COVID [13]. Similar results were presented by Francone: fibrotic traces visible on a CT scan were found in 40.8% (53 out of 130) early disease phase and in 53.6% (70 out of 130) at a later stage [14]. According to Vasarmidi [15] and Rai [16], the rate of COVID-induced fibrosis may exceed 30%.
Currently, there is an active discussion about the possibility of spontaneous resolution and the need for drug treatment of persistent fibrotic lung injuries in post-COVID patients. Studies carried out on a number of respiratory infections such as influenza and atypical pneumonia confirm a clear link between the viral damage to lung tissues, the development of an aberrant inflammatory response, the formation of permanent lesions, and the occurrence of fibrosis [17]. In particular, the presence of persistent COVID-induced fibrotic lung damage one year after COVID is associated with a severe respiratory pathology and concomitant symptoms [5], and at an earlier stage (3–5 months after COVID), with inflammatory response complications in the respiratory tract tissue [18][19][20]. It is plausible that the prolonged inflammatory response causes further damage to the vascular endothelium and epithelium of the respiratory tract and leads to cytokine-induced tissue damage. In turn, this may prevent the restoration of normal respiration and lung tissue regeneration [18]. The upregulation of TGF-β1 and other growth factors (FGF, EGF), followed by the activation of profibrotic pathways and the renin-angiotensin system imbalance, may also contribute to the development of post-COVID lung fibrosis [21].
The dynamics of the long-lasting sequelae in patients who have recovered from severe COVID indicate that there is a 30% chance of developing a persistent respiratory system pathology and a 10% chance of developing a severe pathology. This includes the severe disruption of respiration, reduction of exercise tolerance, and the concomitant development of persistent fibrotic lung damage. Unfortunately, to date, there are no data that would allow one to reliably estimate the chances of the pathology progression and the development of COVID-induced lung fibrosis 10 years after COVID recovery. Therefore, it is reasonable to forecast the future rate of COVID-induced lung fibrosis using the data of the patients who have recovered from other coronavirus infections such as SARS. More importantly, SARS and COVID have similar pathogenetic features, analogous dynamics in the resolution of the sequelae during the first 12 months after diagnosis as well as comparable rates of respiratory pathologies (23.7% for SARS and 33% for COVID) and persistent fibrotic lung damage (27.8% for SARS and 38% for COVID) [22][23]. Based on the 15 years of observational data on SARS patients, it has been established that the severity and prevalence of the respiratory pathology and the residual lung damage are reduced within the first 12 months after recovery [22][24], but remain unchanged in the next 14 years [25]. Another set of observational data allows one to conclude that the risk of developing fibrosis after recovery from a SARS infection is ca. 20% [22][23][25][26][27]. Thus, for patients who have recovered from moderate to severe COVID, we can estimate the risk of developing fibrosis at 2–6%. In turn, this means that the estimate for the prevalence of COVID-induced fibrosis is somewhere between 10 and 15 patients per 10,000 people in the general population, which is 10–100 times higher than the risk of idiopathic lung fibrosis.
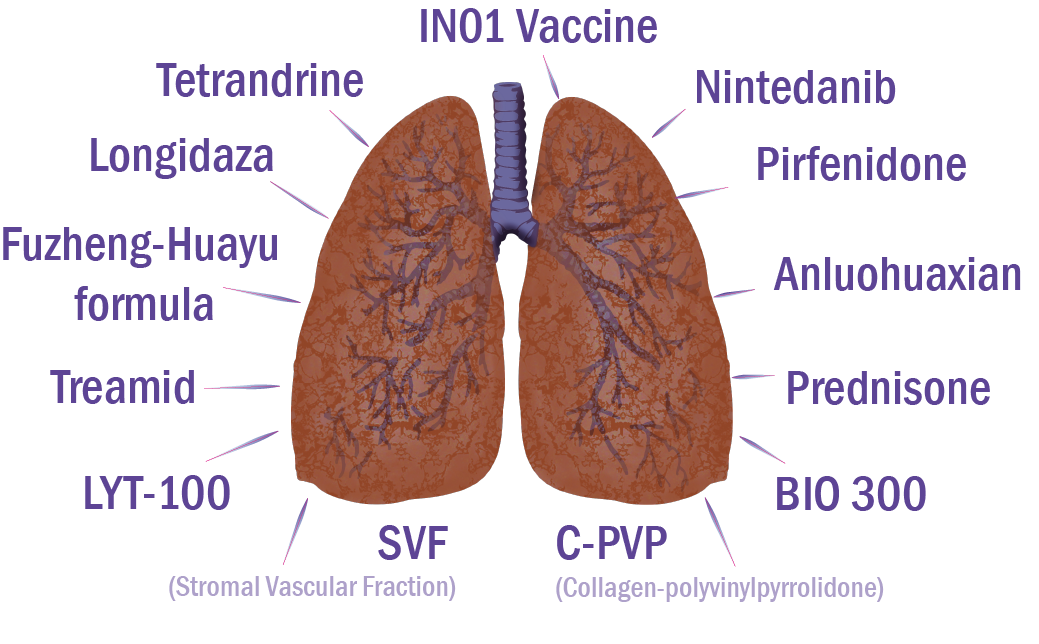
More detailed information on drugs in clinical trials for the treatment of lung fibrosis associated with COVID is provided below.
Phase III trial of nintedanib began in October 2020 [28]. The study included patients 18–89 years old and verified to have had COVID (positive PCR or serologic test results within the previous 2–6 months), with radiological signs (CT) of fibrosis (>10% of lung capacity) and DLCO ≤ 70%. The primary objective is to assess the efficacy of nintedanib in the retardation of lung fibrosis progression in COVID survivors expressed as FVC levels in 12 months compared to placebo. The authors aim to compare the rate of DLCO decreased after 6 and 12 months, exercise tolerance after 12 months, the increase in fibrotic changes (HRCT) after 12 months, and health-related quality-of-life changes; to evaluate dyspnea dynamics, changes in depression and anxiety levels, biomarkers of lung damage, lung hypertension and inflammation, rate of lung hypertension after 12 months compared to the moment of inclusion; to assess the link between genetic predisposition (MUC5B polymorphism) and lung fibrosis in COVID survivors, and the safety of the compound.
Thus, corticosteroids may improve the symptoms of post-COVID pulmonary fibrosis by decreasing inflammation in the lungs. Confirming this hypothesis, a case series of three post-COVID patients revealed that a 1-month course of prednisone treatment resulted in a mild clinical improvement (reduced oxygen consumption at home, improvement on chest X-ray scans) with no major adverse effects [29]. Moreover, steroids are the accepted first-line treatment of organizing pneumonia, which was shown to be the prevailing condition in post-COVID patients having interstitial lung disease, with significant functional deficit 6 weeks after discharge (4.8%, 35 out of 837 survivors) [30]. Thirty of these patients received prednisone, resulting in significant symptomatic and radiological improvement.
Treamid caused anti-inflammatory and antifibrotic effects and lung tissue regeneration in animals with experimental pulmonary fibrosis by suppressing production and deposition of collagen and stimulating endothelial progenitor cell synthesis. Preclinical data suggest that Treamid efficiently suppresses inflammation. These results are promising for the application of this drug as an antifibrotic therapeutic and the restoration of the structure and function of lungs in patients suffering from IPF and novel COVID-induced fibrosis fibrosis [31]. Clinical trials of Treamid started in September 2020 and are aimed at the evaluation of the safety and rehabilitation efficacy of the medication in patients who recovered from COVID-induced pneumonia [32].
A clinical trial of bovhyaluronidase azoximer efficacy and safety for the prevention and treatment of post-inflammatory fibrosis and interstitial lung disease after COVID started in June 2020 [33]. The objective of the study is to compare the outcomes related to post-inflammatory fibrosis and interstitial lung disease in adults with post-COVID pulmonary complications in the group receiving bovhyaluronidase azoximer for treatment or prevention, and in the group of patients under dynamic observation. This trial includes patients aged over 18 years, with residual pulmonary changes due to COVID complications, discovered no later than 2 months from disease onset [33]. Primary trial efficacy endpoints involve the assessment of the fibrotic lung tissue damage and interstitial changes evidenced by the HRCT data at 2.5 months compared to the initial values. Secondary endpoints include lung fibrosis and interstitial changes, ground-glass opacity, hydrothorax, consolidation of images analyzed by Botkin. AI software (artificial intelligence); FVC and DLCO changes, rate of dyspnea according to mMRC scale, SpO 2, 6MWT data, SpO 2 dynamics after 6MWT; changes in residual and total lung capacity [33].
The novel coronavirus-induced disease led to a pandemic that poses a global threat to human health. The monitoring of patients who have recovered from COVID-associated pneumonia demonstrates that the significant reduction in DLCO and associated fibrotic signs in the lung parenchyma are factors associated with a negative prognosis. The persistent respiratory complications may cause substantial population morbidity, long-term disability, and even death due to lung fibrosis progression. The incidence rate of post-COVID lung fibrosis can be estimated at 2–6% after a moderate illness. According to this estimation, the prevalence of post-COVID lung fibrosis will be from 10 to 30 patients per 10,000 populations, which is 30 times higher than the IPF prevalence.
The medical community is currently facing a lack of efficacious treatment options for COVID-induced fibrosis and is therefore compelled to look at the repurposing and repositioning of drugs and drug candidates. We believe that anti-inflammatory treatment for 6 months after COVID-associated pneumonia will reduce residual lung inflammation and improve the impaired diffusing capacity of the lungs. In turn, this will boost lung tissue regeneration and prevent persistent respiratory pathology. We hope that the clinical trials presented above will result in the discovery of efficacious tools to combat pulmonary fibrosis, one of the major COVID complications.