In age-accelerated SAMP8 mouse model, GKM3 groups showed significantly increased latency in the passive avoidance test and time of successful avoidance in the active avoidance test. The TBARS and 8-OHdG from the aged mice brains also showed a significant reduction in the groups treated with GKM3. In addition, lower accumulation of the amyloid-β protein was found in SAMP8 mice brains with the supplement of GKM3. These results indicated that L. plantarum GKM3 delayed the process of aging, alleviated age-related cognitive impairment, and reduced oxidative stress.
1. Introduction
It has been an issue that population-suffered cognitive impairment has become bigger following the growth of the amount of older individuals in the last decade
[1]. According to the report from the World Health Organization (WHO), the number of people with cognitive decline, which was more than 46.8 million in 2015, will reach over 74.7 million in 2030, which is almost increased by half within fifteen years
[2]. Aging, recognized as a strong correlation with cognitive impairment, is generally thought to be associated with oxidative stress, which is involved in the accumulation of free radicals and promoted many physiological dysfunctions especially in the brain, an oxygen-sensitive organ, leading to degenerative symptoms such as memory loss and decline of learning ability
[3][4][5].
As aging is inevitable and its relative negative symptoms are complicated, people pursue treatments for anti-aging, ranging from diet therapies to more than drug treatments
[6][7]. For example, ginseng (
Panax quinquefolius) root extract demonstrated improved cognitive function and was marked as a favorite by the Koreans
[8].
Ginkgo biloba extract, another example, was loved by the Japanese for its functions in cognitive enhancing and oxidation-reduction
[9]. Although many reports have suggested that the plant-based diets provide antioxidant effects and retard the aging process, there are more interesting focuses on the gut microbiota or even on a group of specific bacteria in the gut that could metabolize these nutraceuticals and provide positive multi-modulation effects to the host, including longevity
[10][11][12][13][14]. Gut bacteria could produce short-chain fatty acids (SCFAs) such as acetate, propionate, or butyrate from one’s diet
[15]. Children who consumed plant-based enteral nutrition showed higher SCFAs than the normal kids in their stool samples
[16]. These SCFAs can play an important role in the human gastrointestinal epithelium
[17]. Acetate can cross the blood-brain barrier, inducing hypothalamic neuronal activation
[18]. In addition, butyrate has been studied regarding the effect of suppressing colonic inflammation in the human gut
[19]. Therefore, it matters what kind of microbiota or specific bacteria is in the human gut, especially in terms of influencing body health and longevity
[20][21].
The intestinal microbiota also can impact the gut-brain axis and delay the process of aging, thereby preventing the progress of age-related diseases such as amnesia, dementia, or even Alzheimer’s disease
[22][23]. The neurotransmitter gamma-aminobutyric acid (GABA), for example, could be produced by certain bacteria strains that have been reported with the proliferation of epithelial stem cells in the gut and the improvement of depressive-like behavior in mice
[24][25]. With current supportive data, the addition of probiotics as a prevention method has been a trend for these age-related neurological disorders
[26][27].
Lactobacillus is the most common probiotic that showed a high possibility of developing functional food as it has existed in human food for a long time
[28]. However, individual bacterial species have unique bioactivities, from strains to strains, that require experimental confirmation. In our previous studies, we isolated the probiotic strain
Lactobacillus plantarum GKM3 from pickled leaf (
Brassica juncea), also known as the longevity vegetable in Taiwanese Hakka society, and conducted functional studies on boosting the gastrointestinal tract and immunity in vivo
[29][30][31]. Therefore, we expected the likelihood of
L. plantarum GKM3 alleviating the process of aging and age-related cognitive impairment.
2. Development and Findings
There were no significant difference in the body weight or food intake between the control group and the probiotic groups (Table 1). This indicates that the probiotic GKM3 is safe and non-toxic to the mammal. Under the same metabolic parameter and energy consumption, the effects of anti-aging in GKM3-fed SAMP8 can be discussed in the following section.
Table 1. Body weight, food intake, and water consumption.
| Sex |
Group |
Body Weight (g) |
Food Intake (g/day) |
Water Consumption (mL/day) |
Initial
(Week 0) |
Final
(Week 14th) |
Gain |
| Male |
Control |
28.37 ± 1.03 a |
32.06 ± 1.05 b |
3.69 ± 0.47 c |
4.79 ± 0.06 d |
6.67 ± 0.10 e |
| GKM3-L |
28.81 ± 0.78 a |
31.14 ± 1.01 b |
2.33 ± 0.36 c |
4.74 ± 0.07 d |
6.65 ± 0.16 e |
| GKM3-H |
28.58 ± 0.76 a |
32.12 ± 1.03 b |
3.55 ± 0.57 c |
4.71 ± 0.08 d |
6.53 ± 0.15 e |
| Female |
Control |
27.14 ± 0.95 f |
27.61 ± 0.87 g |
0.47 ± 0.61 h |
4.09 ± 0.04 i |
4.64 ± 0.14 j |
| GKM3-L |
27.01 ± 0.73 f |
28.05 ± 0.85 g |
1.49 ± 0.70 h |
3.98 ± 0.11 i |
4.60 ± 0.06 j |
| GKM3-H |
26.63 ± 0.39 f |
28.51 ± 0.84 g |
1.88 ± 1.02 h |
4.10 ± 0.14 i |
4.69 ± 0.07 j |
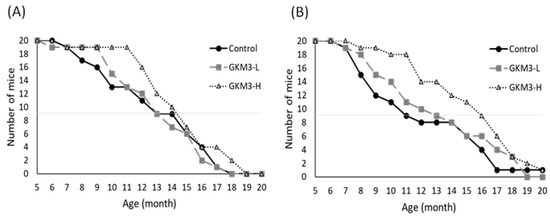
Through observation of the lifespan of SAMP8, we note the death started from 6–7 months; however, both males and females in the probiotic GKM3-H group tended to present low death rates (
Figure 1). Especially up to 11 months, the survival rates were very different. The GKM3-H group still kept 90–95% of the survival rate but the control group only presented 60–40% of the survival rate. Compared with other reported nutrients such as plant extract or marine sources on anti-aging effect, probiotic GKM3 provided a better result especially in terms of a longer survival rate
[32][33]. It is possible that those anti-aging materials contained lipid, organic acid, polyphenols, or vitamins, which also can be easily found from the probiotic’s fermentation, and resulted in a longer lifespan
[34][35][36].
Figure 1. The lifespan of SAMP8 mice in males (A) and females (B). GKM3-L and GKM3-H were represent the groups fed probiotic GKM3 at the low dosage of 1.0 × 109 CFU/kg BW/day and the high dosage of 5.1 × 109 CFU/kg BW/day, respectively (n = 10)
Behavior avoidance tests are usually used for evaluating learning and memory in subjects. Memory is defined as a behavioral change caused by an experience, while learning is defined as a process for acquiring memory
[37]. Both memory and learning were formed or achieved by being involved with nerve transmission and the nerve cell. Hence, the process of aging could increase the accumulation of ROS in neurons and damage the cell, resulting in poor memory and learning ability
[38]. The passive avoidance test is a fear-motivated test in which subjects are required to inhibit a previously exhibited response
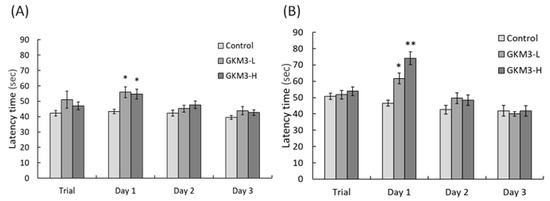
[39]. Mouse with a better memory and learning ability can avoid entering dangerous areas. Conversely, active avoidance requires subjects to emit a response such as running to a safe place to avoid a noxious stimuli. Good memory and learning skills help the mice respond to alerting events and avoid incoming dangers. Evidence from our study showed that probiotic GKM3 could contribute to learning and memory by inhibiting the occurrence of undesirable responses, while there was no contribution in the control group even after the training procedure (
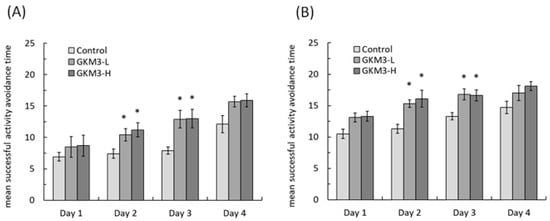
Figure 2). Interestingly, the control group showed an increased tendency in the successful avoidance followed by the training days in the active avoidance test; however, the group fed with probiotic GKM3 showed a stronger significant successful avoidance (
Figure 3). This could be explained by the trial-designed base
[40]. Mice were forced to learn what to do in the active avoidance test but were relatively unstimulated in being required to learn what not to do in the passive avoidance test. In spite of the different reactions presented by the control mice, the effect of probiotic GKM3 in improving learning and memory could not be denied with the stimulations of both different mechanisms
[41].
Figure 2. Passive avoidance ability of male (A) and female (B) SAMP8 mice. Values are presented as mean ± S.E.M. (n = 10). * p < 0.05 and ** p < 0.01 were regarded as a significant difference (one-way ANOVA). GKM3-L: 1.0 × 109 CFU/kg BW/day (low dose); GKM3-H: 5.1 × 109 CFU/kg BW/day (high dose).
Figure 3. Active avoidance ability of male (A) and female (B) SAMP8 mice. Values are presented as mean ± S.E.M. (n = 10). * p <0.05 was regarded as a significant difference (one-way ANOVA). GKM3-L: 1.0 × 109 CFU/kg BW/day (low dose); GKM3-H: 5.1 × 109 CFU/kg BW/day (high dose).
Similar behavior results were reported by Yong et al. with chicken extract as a diet addition and Su et al. with the supplementation of yam for SAMP8 mice
[40][41]. As the bioactive compounds should exhibit a tremendous difference between the meat extract and plant origin, it could be speculated that the effect of cognitive maintenance was highly related to the alteration of gut microbiota
[42][43]. Probiotics can produce metabolic compounds that enhance or suppress the growth of certain gut microorganisms
[44]. These metabolic compounds, such as peptides, cortisol, or SCFAs, can also modulate the nervous system and maintain brain functions through the gut-microbiome-brain interaction. It was found that the gut microbiota in centenarians was very different from the aging population
[45]. In particular, the relative abundance of
Firmicutes was found. Even though we did not analyze the microbiota in this study, several papers pointed out that the administration of probiotics altered the gut microbiota
[46][47][48][49][50]. In addition, our unrevealed data concerning a probiotic mixture maily contained
L. plantarum GKM3 in a clinical trial that showed an increase of several
Bifidobacterium species and several
Lactobacillus species in the stool analysis after four weeks of consumption. It is suggested that
L. plantarum GKM3 may maintain brain functions by changing the composition of gut microbial flora
[51].
SAMP8 is a neuropathological model of accelerated brain aging derived from an AKR/J breeding colony by Professor Toshio Takeda at the Kyoto University
[52]. Regarding age-associated morphological alteration, early amyloid accumulation in the hippocampus was found in SAMP8 mice, which resulted in learning disturbances and impaired memory
[53]. Aβ was considered to induce ROS formation, lipid peroxidation, and neurotoxicity in hippocampal neurons
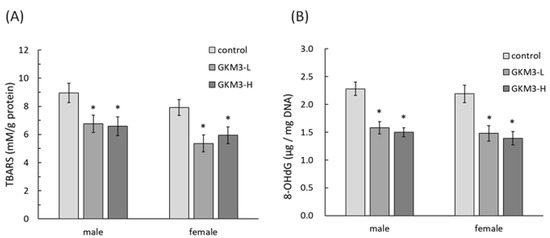
[54]. Our results reveal that probiotic GKM3 not only inhibited oxidative stress in the brain (
Figure 4) but also was involved in the upper inhibition of the amyloid formation (
Figure 5). TBARS is formed as a by-product of lipid peroxidation and MDA is formed as its end-product. 8-OHdG is a common end-product of deoxyribonucleic acid (DNA) oxidation. That is, high levels of TBARS and 8-OHdG both represent strong oxidation and result in cognitive impairment
[55].
Figure 4. The TBARS (A) and 8-OHdG (B) level in SAMP8 mice brains after 14 weeks of probiotic GKM3 consumption. Values are presented as mean ± S.E.M. (n = 5). * p < 0.05 was regarded as a significant difference (one-way ANOVA). GKM3-L: 1.0 × 109 CFU/kg BW/day (low dose); GKM3-H: 5.1 × 109 CFU/kg BW/day (high dose). Abbreviations: TBARS, thiobarbituric acid reactive substances and 8-OHdG, 8-hydroxy-2′-deoxyguanosine.
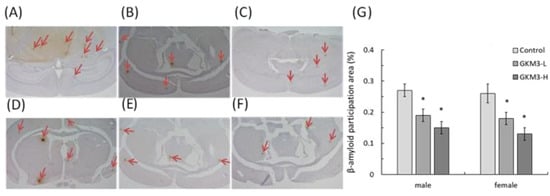
Figure 5. The β-amyloid participation in SAMP8 mice brains (40X) after 14 weeks of probiotic GKM3 consumption. (A): control male; (B): GKM3 low-dose male; (C): GKM3 high-dose male; (D): control female; (E): GKM3 low-dose female; (F): GKM3 high-dose female; and (G): the percentage of the amyloid-β participation area. The arrows point to the participation of amyloid-β. Values are presented as mean ± S.E.M. (n = 5). * p < 0.05 was regarded as a significant difference (one-way ANOVA).
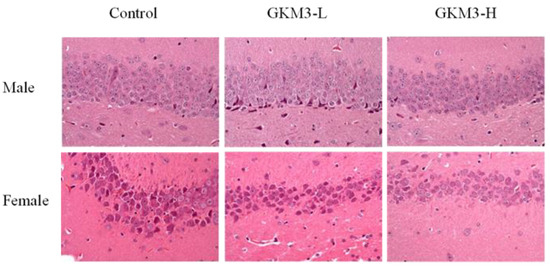
Pyramidal cells, a type of populous neuron involved with the sensory and motor cues in the hippocampus, could contribute to information processing, learning, and memory
[56]. The disordered arrangements of pyramidal cells in the hippocampal CA1 region were found in Alzheimer’s disease-affected mice
[57]. It is indicated that
L. plantarum GKM3 could alleviate the functional decline of neuron-transmitting by maintaining cell morphology (
Figure 6). That is, long-term administration of probiotic GKM3 could enhance a better consciousness and encourage appropriate actions in life.
Figure 6. The hematoxylin and eosin staining of the hippocampus area in SAMP8 mice brains after 14 weeks of probiotic GKM3 consumption. GKM3-L: 1.0 × 109 CFU/kg BW/day (low dose); GKM3-H: 5.1 × 109 CFU/kg BW/day (high dose).
3. Conclusions
In this study, we examined the dose-dependent effect of long-term administration of L. plantarum GKM3 on longevity in both male and female SAMP8 mice. In addition, supplementation of probiotic GKM3 showed the improvement of memory and learning ability by being involved in anti-oxidative stress, by lowering Aβ accumulation, and by maintaining the arrangement of nerve cells in the hippocampus. These results suggest that probiotic L. plantarum GKM3 could act as an antioxidant for delaying the aging process and prevent age-related cognitive impairment. With its desirable functions and safe consumption history, L. plantarum GKM3 is a promising probiotic supplementation for the elderly.