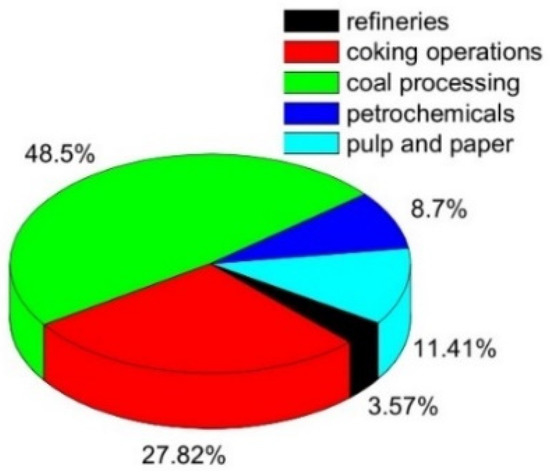
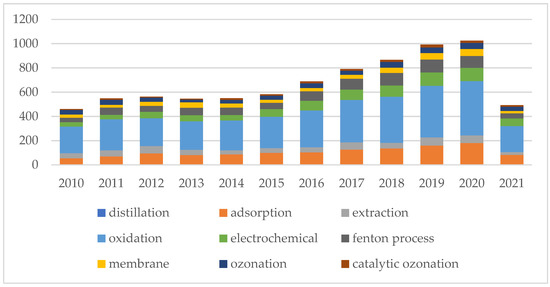
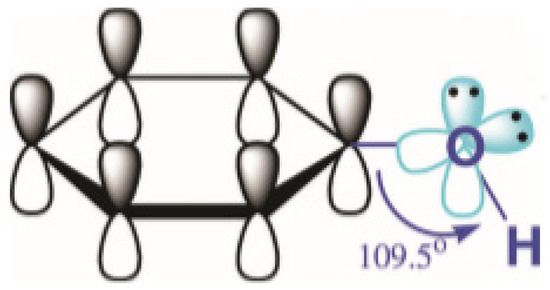
Phenol is one of the top priority contaminants and also a potentially carcinogenic pollutant
[13]. Phenol is a toxic, carcinogenic, mutagenic, and teratogenic substance that can inhibit the rate of biodegradation at concentrations of more than 50 mg/L in wastewater
[7]. For human and aquatic life, the levels of toxicity vary between 9 and 25 mg/L. Exposure to phenolic compounds can cause eating disorders, weight loss, diarrhea, vertigo, salivation, and dark coloration of feces
[15]. According to the Health Protection Agency (HPA), there is about 60–88% of phenol exposure through inhalation and followed by oral and dermal exposure
[16]. The toxicological properties of phenol have been contributed mainly by the formation of organic species and free radical species and their hydrophobicity. The structure of phenol itself shows its reactivity, which leads to its properties such as persistence in the environment, toxicity, and possible carcinogenicity to living organisms
[17]. The presence of phenol in wastewater during disinfection and oxidation processes can also form substituted compounds and toxic intermediates as secondary pollutants that can inhibit microorganisms in biological treatment processes
[14]. Therefore, the removal and mineralization of phenolic compounds from wastewater is necessary before being discharged into the environment. Consequently, wastewater treatment with phenol species has attracted a lot of attention due to the toxicity and low biodegradability of phenolic compounds.
Phenol acts as a harmful pollutant even in very low concentrations in water. Several methods have been developed to remove phenolic compounds from wastewater. Chemical adsorption and oxidation are the most common methods, but they still have some drawbacks including requiring higher energy consumption, maintenance (corrosion, sediment, and scale), high operating cost, and being unsustainable. As an alternative, ozone-based technology has been widely used to degrade organic pollutants because ozone has a high oxidizing ability. However, single ozonation exhibits very low efficiency, requires a large amount of ozone, low solubility in water, low reaction rate with organic compounds, and slow mineralization rate. Therefore, to overcome this problem, the involvement of a catalyst in the ozonation process is able to increase the degradation efficiency and reduce the use of ozone because there are reactive oxygen species formed from the decomposition of ozone involved in the reaction (i.e., hydroxyl radicals).
Catalytic ozonation consists of homogeneous catalytic ozonation and heterogeneous catalytic ozonation. Heterogeneous catalytic ozonation has the tremendous advantage of no secondary pollutant produced and an easier catalyst regeneration process than homogeneous catalytic ozonation. The degradation of phenol in solution using heterogeneous catalytic ozonation processes has been developed by many researchers. Of the various types of catalysts that have been developed, zeolite has the potential to be a promising catalyst in the catalytic ozonation process for the degradation of phenol waste. Zeolites have a large specific surface area, good thermal and chemical stability, and controllable hydrophilic/hydrophobic properties. Several parameters need to be reviewed to produce an ideal zeolite for the catalytic ozonation process of phenol waste removal, namely the Si/Al ratio, suitable pore size for adsorption of phenolic compounds, morphology, acidity level, and specific surface area. Furthermore, with a large surface area and short diffusion path length, nano-sized zeolites provide an active site that is more accessible to the pollutants so that the production of nano-sized zeolites is currently being developed.
There is still a lack of research with regard to the use of heterogeneous catalytic ozonation for phenol removals. Thus, to fulfill this research gap, the following strategies can be set for the future directions:
- (i)
-
Necessities of standardized performance evaluation.
In the literature, the choice of reactor type configuration is well reported to play a role in determining phenol removal efficiency. In fact, the number of parameters such as operating testing conditions (pH, ozone concentration and flow rate, temperature, and pressure) and reactor configuration greatly affect the catalytic performance. As a result, we propose the necessity of a standardized performance evaluation technique so that the different catalysts developed for phenol removal can be appropriately compared.
- (ii)
-
Development of zeolite-based catalyst for phenol removal
Most research in heterogeneous catalytic ozonation for phenol removal has been to develop metal oxides-based catalysts. Furthermore, developing an advanced synthetic method, that is, ultrafast synthesis using continuous flow to synthesis various types of zeolites in the order of seconds, provides great potential to facilitate the large-scale production of zeolite.
- (iii)
-
Elucidation of active sites in zeolite-based catalyst
Besides the mass production of zeolite, the understanding of fundamental reaction mechanisms, in particular, the catalytic active sites in heterogeneous catalytic ozonation are not fully understood. Thus, the utilization of in situ and operando characterizations will be beneficial to fully understand the active sites especially in zeolite-based catalysts for phenol removal.
- (iv)
-
Application of artificial intelligence (AI) for catalyst development
The development of an ideal catalyst for heterogeneous catalytic ozonation for phenol removal is still an important issue. An automatic machine learning framework based on artificial intelligence is advocated to allow the development of high-throughput catalysts with desired physiochemical properties for phenol removal in large-scale applications.
- (v)
-
Techno-economic evaluation of heterogeneous catalytic ozonation
After finding the most optimum catalyst and suitable synthetic methods as well as reactor configuration process, techno-economic evaluation is necessary to be conducted in order to analyze the economic performance of the implementation of an industrial process. Ultimately, heterogeneous catalytic ozonation technology supported with the ease of the recovery and reuse of catalyst will lead to the creation of green, sustainable, environmentally friendly technology and will be the embodiment of the circular economy concept.