1000/1000
Hot
Most Recent

Diabetic macular oedema (DMO) is one of the leading causes of vision loss associated with diabetic retinopathy (DR). New insights in managing this condition have changed the paradigm in its treatment, with intravitreal injections of antivascular endothelial growth factor (anti-VEGF) having become the standard therapy for DMO worldwide. However, there is no single standard therapy for all patients DMO refractory to anti-VEGF treatment; thus, further investigation is still needed.
Diabetic retinopathy (DR) is one of the feared chronic complications of Diabetes Mellitus (DM), a pandemic disease that affects approximately 463 million adults nowadays. Its prevalence has been rising in recent years, exemplifying its steady increase; by 2045, 700 million adults are expected to live with diabetes. A total of 40% of diabetic patients over 40 years old suffer from DR. [1].
Diabetic macular oedema (DMO) is associated with an abnormal increase in fluid volume in the macula, whether infiltrating retinal layers or collecting in the subretinal space. It is a significant cause of vision loss in those patients since it primarily affects central vision. DMO may occur in any disease stage and is more frequent in insulin-dependent type 2 DM [1].
The pathogenesis of DMO is multifactorial and complex, with a combination of pathological conditions related to retinal hypoxia, vascular permeability, angiogenesis, and inflammation processes leading to the development of the characteristic retinal alterations in patients with DMO [2][3][4][5].
Since the 1970s, focal/grid laser treatment has been considered the only available treatment for the management of this and it was accepted that in general, it only stabilised functional loss but did not improve it [6]. However, the breakthrough of intravitreal antivascular endothelial growth factor (VEGF) therapy has now replaced laser treatment as several trials have shown improved outcomes [7][8], which is nowadays the treatment of choice. However, anti-VEGF therapy has several limitations since it requires multiple visits and injections, increasing the burden placed on the healthcare system. Furthermore, DMO persists in more than 40% of patients even after numerous intravitreal injections according to the protocol I trial [4]. The efficacy of anti-VEGF treatment may be less pronounced in the clinical practice setting where undertreatment is very frequent [9][10][11] ( Figure 1 ). Moreover, there are certain doubts about the long-term effect of the inhibition of a factor (VEGF) that is essential, among other things, to keep the choriocapillaris in good condition.
The inner BRB is composed of endothelial cells of retinal vessels sealed by tight junctions, where pericytes also seem to play an important role, and the astrocytes create a complex structure formed by the interaction of different scaffolding and transmembrane and signalling proteins [2][12][13].
As mentioned, the inner BRB is highly regulated by a complex neuro-glio-vascular system made by endothelial cells and their basal lamina, surrounded by pericytes, astrocytes, Müller cells and microglia, which interact with them, and other retinal components selectively controlling the molecular transport across this barrier [2][14][15].
BRB breakdown can result from a disruption of the tight junctions, by upregulation of substance transport across RPE cells or retinal vascular endothelial cells, by degenerative changes to the barrier-forming cells or to the regulatory cells (pericytes and glia cells) [16]. BRB breakdown can be related to localized structural defects, such as microaneurysm, or to a diffuse retinal vascular leakage in which diffusible factors can be involved.
Capillary abnormalities and obstruction in DR induce retinal hypoxia, which is also one of the hinges of DME pathogenesis [2][17]. In hypoxic conditions, hypoxia-inducible factor 1α (HIF-1α) promotes the expression of angiogenic factors, including VEGF. Therefore, HIF production promotes retinal vessels formation in an attempt to improve retinal oxygenation but contributing to pro and antiangiogenic factors disbalance and its deleterious consequences for retinal cells and BRBs [2][14][18][19]. VEGF and HIF-1α have shown an intravitreal concentration up to 10 times higher in diabetic patients than in nondiabetic ones [17][14].
Choosing between different anti-VEGF therapies to initiate DMO treatment requires the consideration of many factors, including cost and efficacy [20]. Switching strategy implementation must be taken into account in cases unresponsive to the initial regime with a monthly intravitreal antiangiogenic drug at three or six months follow-up when either a persistent CMT on OCT, a visual acuity under 20/40, or an improvement of VA of less than a line from baseline, is present [21].
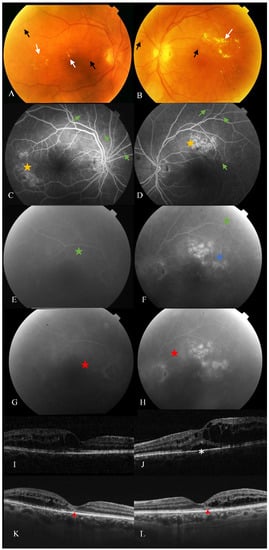
A non-negligible percentage of DMO patients do not respond to antiangiogenic treatment, although there is a lack of consensus regarding when to consider a DMO to be persistent, resistant or refractory [22][23][24]. For example, DRCRN and RISE/RIDE papers defined persistent DMO as those eyes under monthly intravitreal treatment for at least 6 months with a central subfield thickness (CST) measured by OCT of above 250 µm [8][22]. In contrast, other research groups considered refractory DMO to occur when there was no response to the last three monthly anti-VEGF injections, considering a lack of response to be a worsening of BCVA according to two early treatment diabetic retinopathy studies (ETDRS) or when there was a reduction of less than 10% or 50 µm in CST [25][23][26][27].
Whether one criterion or the other is considered, resistant or persistent DMO is still considered a relevant proportion of DMO patients. In protocol T from DRCRN, 40% of patients suffer from persistent DMO within 24 weeks of treatment [22][28], and 30–40% of patients did not improve visual function in three years of therapy in the RISE/RIDE study [29]. In this framework, from the 1970s, intravitreal corticosteroid treatment has been studied in DMO with positive outcomes due to the potential role of inflammation on its pathogenesis and because some of them seem to also have anti-VEGF properties [30][31][32]. Triamcinolone acetonide (TA) was the first corticosteroid used for this purpose [33].
Bearing in mind the inflammatory component in DMO, corticosteroids, either used alone or in combination with specific anti-VEGF therapy, are a current therapy that can help us individualise treatments [33][20][26]. Intravitreal dexamethasone implant (0.7 mg) (Ozurdex ® , Allergan, Inc., Irvine, CA, USA) treatment has been demonstrated to produce an improvement in structural parameters, mainly CMT and CST, alone or in combination with anti-VEGF therapy [25][23][26][32][34]. Its posology is more convenient since it consists of a biodegradable solid polymer drug-delivery system whose efficacy has been demonstrated up to six months after its administration [31][35]. Fluocinolone acetonide (FA) (0.19 mg) is the content of another nonbiodegradable implant (Iluvien ® , Alimera Sciences, Alpharetta, GA, USA) which can release the drug for up to three years, and FAME studies demonstrated its efficacy particularly in long-term chronic DMO [36][33].
As mentioned, inflammatory factors play a key role in DMO pathogenesis, and their own signalling pathways and expression are influenced by Rho kinases. In vitro assays have also been conducted to prove this relationship using human retinal Müller glial cells and microvascular endothelial cells, ex vivo retinal explants, bovine retinal endothelial cells and rat retinal cells [37][38][39][40][41]. Results of recent research indicate that ROCK-1 activation induces focal vascular constrictions, endoluminal blebbing, retinal hypoxia, and remodelling of RPE cells contributing to outer barrier breakdown [37][42]. The blebbing-induced closure reversed by ROCK inhibitors, could open a window for intervention in case of macular ischemia.
Its contribution also has been demonstrated in simvastatin-induced nitric-oxide-mediated dilatation of retinal arterioles [43]. The eNOS enzyme and its phosphorylation appear to be downregulated in retinal endothelial cells, and fasudil treatment also has been proved to have a positive effect in reversing the decreased expression of this enzyme, but the production of nitric oxide (NO) is required to be maintained for fasudil to work [44].
ROCK-1 translocation and blebbing and an increased level of activated Rho in endothelial cells were also found in diabetic rat retinas producing endothelial impairment and the DMO [42][44]. Rat and human models have shown that under hyperglycaemic conditions and ROCK-1 activation, which occurred in the RPE, occludin, ZO-1 and claudin-5 protein levels are reduced, destabilising tight junctions in endothelial cells and causing inner-BRB disruption [9][45].
The eNOS enzyme and its phosphorylation appear to be downregulated in diabetic retinal endothelial cells, and fasudil treatment has also proven to have a positive effect in reversing the decreased expression of this enzyme, but the production of nitric oxide (NO) is required to be maintained for fasudil to work [46][47][48][49][50][51][52].