2. Gelatin and Collagen
Collagen belongs to the native extracellular matrix (ECM) of the human body. The ECM is responsible for providing the mechanical structure of tissues, as well as for wound healing and controlling other cellular processes
[24]. Thus, collagen belongs to the highly interesting materials for tissue engineering. Gelatin, which is derived from collagen by partial hydrolysis, has partly similar and partly different physical properties, varying depending on the origin and processing of the material
[25][26].
As usual for many biopolymers, collagen and gelatin are water-soluble and thus need a crosslinking step
[27]. Alternatively, the water-resistance and mechanical properties can be enhanced by blending them with water-stable polymers. This is why many studies found in the literature report on blends of collagen or gelatin with other polymers.
For example, Chen et al. prepared polyurethane/collagen core-shell nanofibers by coaxial electrospinning that were crosslinked by glutaraldehyde (GA) vapor
[28]. Thermoplastic polyurethanes (TPUs) are known to have not only good mechanical properties, but also good biocompatibility, resulting in their frequent use in implants, catheters, etc.
[29]. Special medical-grade TPU can additionally avoid in vivo degradation
[30]. The coaxial electrospinning technique used here allows spinning two different polymers through the inner and the outer part of the needle, driven by two independent syringe pumps and fed from separate reservoirs
[28]. In comparison with pure collagen, the core-shell fibers showed significantly improved mechanical properties, combined with the highest viability of pig iliac endothelial cells (PIECs) cultured on the different electrospun nanofiber mats, even higher than on pure collagen fibers. All nanofiber mats showed a better cell viability than reference tests on coverslips. The authors mentioned that the fiber diameters, mat porosity, and mechanical properties of the nanofiber mats significantly influenced cell growth and migration, which resulted in compound nanofibers—especially produced by coaxial electrospinning of collagen around TPU—having the best growth conditions. They also mentioned that the coaxially collagen-coated TPU fibers were much more attached cells, while simply coating a pure TPU nanofiber mat macroscopically with collagen did not have the same effect of 3D ingrowth into the scaffold, showing the importance of combining material and structure. Similar results were found by the same group with TPU/collagen blended nanofibers
[31].
A polymer blend from collagen and poly((D,L-lactic)-
co-glycolide) (PLGA) was investigated by Yang et al.
[32]. PLGA belongs to the synthetic biodegradable polymers, which are often studied for tissue engineering applications, but usually show insufficient cell adhesion and proliferation if used purely
[33]. Blending PLGA with collagen, however, led to electrospun nanofiber mats which did not need to be crosslinked to avoid swelling in culture medium and on which human dermal fibroblasts (HDFs) and mice fibroblasts containing green fluorescent protein (GFP) showed a significantly higher viability than on pure PLGA nanofiber mats
[32]. This was attributed to the chemical composition of the matrix material, since the fiber diameters were kept constant in all nanofiber mats. The authors explained that collagen and its integrin-binding domains facilitated the cell attachment for anchorage-dependent cell types as the fibroblasts used in this study. A comparison with tissue culture plates additionally showed much higher cell numbers inside the nanofibrous mats due to the available void volume.
Quite a different approach was chosen by Akhshabi et al., who combined collagen with chondroitin sulfate, a negatively sulfated glycosaminoglycan
[34]. The electrospun collagen/chondroitin nanofiber mat was cross-linked with EDC/NHS (1-ethyl-3-(3-dimethyl-aminopropyl)-1-carbodiimide hydrochloride/
N-hydroxy succinimide), before cytocompatibility was investigated using corneal epithelial cells. Crosslinking was found to significantly increase the biostability of the nanofiber mats, while chondroitin was reported to significantly promote cell proliferation, as compared to pure collagen. This was explained by the role of chondroitin sulfate, which induces skin tissue regeneration
[35], osteoblast adhesion
[36], and fibroblast proliferation
[37].
Due to the chemical similarity of gelatin and collagen, gelatin can be expected to show similar effects in increasing cell adhesion and proliferation. Baiguera et al. used genipin cross-linked gelatin nanofiber mats as the base to include decellularized rat brain extracellular matrix as an active agent, before mesenchymal stromal cells (MSCs) from rat bone marrow were seeded onto these scaffolds
[38]. They found the MSCs grew not only on top of the substrate, but also in the inner volume of the decellularized samples. Seeding these cells on pure gelatin nanofiber mats and those with an additional decellularized rat brain extracellular matrix showed growth on both sides of the nanofiber mats in multilayered cultures, especially for the second tissue. Incorporation of a decellularized rat brain extracellular matrix into gelatin nanofiber mats, however, was found to trigger differentiation of MSCs towards neural/glial precursor cells.
An interesting approach was chosen by Li et al., who blended gelatin with the conductive polymer polyaniline (PAni) to prepare conductive electrospun scaffolds
[39]. With conductive scaffolds, cell attachment, proliferation, migration, and even differentiation can be stimulated
[40]. PAni, on the other hand, is known to be biocompatible
[41] and thus a good candidate to make a nanofiber mat conductive. Li et al. seeded rat cardiac myoblast cells on these electrospun nanofiber mats and found they supported cell attachment and proliferation with different amounts of PAni, as compared to glass and other substrates, but also in comparison with pure gelatin nanofiber mats
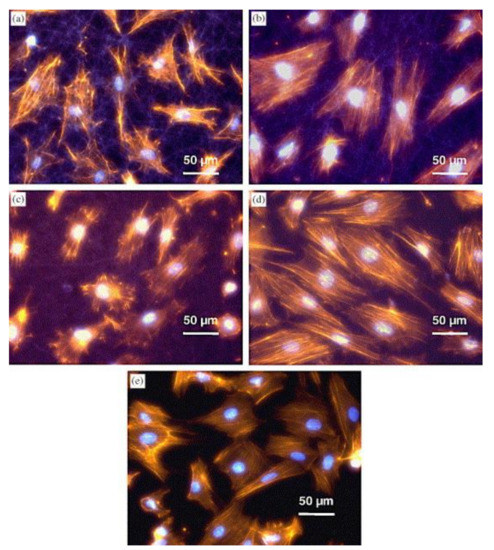
[39]. They found the cell morphology depended on the substrate structure, with cells growing on fibers with larger diameters showing microfilament-rich pseudopodia attached to single fibers (
Figure 1a–c), while cells on thinner fibers were more spread out and exhibited a smooth muscle-like morphology (
Figure 1d), similar to cells grown on glass substrates (
Figure 1e)
[39]. This was attributed to the higher roughness of the fibrous substrates, which provided a larger surface area on which cells could attach, as compared to smooth glass substrates and tissue culture-treated polystyrene, which is commonly regarded as the gold-standard.
Figure 1. Morphology of myoblast cells 20 h after seeding on: (
a) gelatin nanofiber mat; (
b) 15:85 PAni-gelatin blend nanofibers; (
c) 30:70 PAni-gelatin blend nanofibers; (
d) 45:55 PAni-gelatin blend nanofibers; and (
e) glass matrices. Staining for nuclei-bisbenzimide and actin cytoskeleton-phalloidin. Reprinted from
[39], with permission from Elsevier.
Like collagen, gelatin can also be blended with water-resistant or slowly degrading polymers to reduce or avoid crosslinking. Gautam et al. report about PCL/gelatin blended nanofiber mats used for tissue engineering
[42]. They seeded mouse fibroblast cells on nanofiber mats with different blend ratios and found significantly increased cell viability and cell proliferation for the blends as compared to pure PCL scaffolds. This was attributed to the presence of gelatin in these nanofiber mats, which is generally known to result in good cell adhesion and proliferation. Combinations of gelatin with PCL are thus often found in the literature
[43][44][45].
There are, however, many other gelatin blends reported to be advantageous. Comparing PLGA and PLGA/gelatin blended nanofiber mats, Meng et al. found increased cell viability on PLGA/gelatin blends compared to pure PLGA, and in addition they also found better MC3T3-E1 cell proliferation and guidance for aligned nanofibers than for randomly oriented ones
[46]. They mentioned that avoiding crosslinking induced easier cell attachment to the scaffolds, as crosslinking decreased bioactivity and hydrophilicity of the nanofiber mats. Ba Linh et al. investigated polyvinyl alcohol (PVA)/gelatin nanofiber mats after physical crosslinking by methanol and reported osteoblasts to attach firmly on these scaffolds, facilitated by gelatin and the crosslinking process
[47]. Moreover, the hydrophilic nature of PVA and gelatin, as well as the interconnected porous structure of the nanofiber mats, were found to be advantageous for cell proliferation and multilayer growth. A special polyurethane “Tecophilic” was chosen by Vatankhah et al. as a blend partner for gelatin to produce tubular scaffolds for blood vessels, which showed good mechanical properties, higher cell viability and proliferation than pure polyurethane, and good compatibility with blood
[48]. Here again, a deep cellular infiltration into the nanofibrous scaffold was found, which would be impossible on the common flat surfaces, allowing for a larger number of attached cells per area. In addition, triple-blends including gelatin were tested for tissue engineering applications
[49][50][51].
3. Chitosan and Chitin
Chitin belongs to the highly abundant natural polymers and is mainly found in marine crustaceans, shrimps, and crabs. It is insoluble in common solvents. Chitosan, on the other hand, is an important derivative of chitin, obtained by partial deacetylation of chitin under alkaline conditions or by enzymatic hydrolysis and is soluble in acidic aqueous media
[52].
In spite of the problems in dissolving chitin, it is regularly reported in the literature as a possible substrate material for tissue engineering
[53], since it shows similar properties to glycosaminoglycan, which is part of the ECM
[54]. Noh et al. thus prepared electrospun chitin nanofiber mats
[54]. To dissolve the chitin powder, it was first irradiated by Co
60 γ-irradiation and then dissolved in 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP) for 20 days. Electrospinning resulted in ultrafine nanofibers with an average diameter 163 nm. These nanofiber mats degraded in vitro faster than chitin microfibers, promoted cell attachment and spreading of normal human keratinocytes and fibroblasts better than the chitin microfibers, and after coating with type I collagen also promoted cellular response in all cells under investigation. The authors interpreted these findings as a higher functional activity of collagen-coated chitin nanofibers in terms of cell attachment and spreading for normal human keratinocytes as well as fibroblasts. In a similar manner, Min et al. prepared a chitin solution by γ-irradiation and dissolving the resulting chitin in HFIP
[55]. In addition, they investigated deacetylation for transforming the original chitin matrix into a chitosan matrix.
Most groups, however, combine chitin or its derivatives with other polymers. For example, Shalumon et al. blended the water-soluble carboxymethyl chitin with PVA to prepare an aqueous electrospinning solution
[56]. The resulting nanofiber mats were cross-linked by glutaraldehyde vapor before human mesenchymal stem cells (hMSCs) were seeded on them. The cells were found to attach and spread in the washed nanofiber mats, which supported cell adhesion and proliferation (
Figure 2).
Figure 2. SEM images of hMSCs attached on the surfaces of CMC/PVA scaffolds after (
a) 12 h; (
b) 24 h; and (
c) 48 h of incubation. Reprinted from
[56], with permission from Elsevier.
Pangon et al. prepared chitosan/PVA nanofibers with chitin whiskers in different concentrations from succinic acid/water as solvent
[57]. The resulting nanofiber mats were crosslinked by GA vapor, followed by mineralizing hydroxyapatite onto them by applying ten-fold concentrated simulated body fluid and seeding MC3T3-E1 osteoblast cells. They found an increased cell viability and proliferation due to the addition of the chitin whiskers, as compared to pure chitosan/PVA nanofiber mats. This effect was attributed to the clearly modified surface of the nanofiber mats, which showed a rough, uneven structure due to the hybridization with hydroxyapatite, and resulting in significantly improved cell viability and proliferation.
Electrospun nanocomposites of chitin nanofibrils and PCL were prepared by Ji et al.
[58]. The chitin nanofibrils were dispersed in 2,2,2-trifluoroethanol (TFE) in which PCL could also be dissolved, allowing for spinning nanofibers with different chitin:PCL ratios, which showed strongly decreasing average diameters with increasing chitin content. For high chitin nanofibril contents, the water contact angle was significantly reduced. When seeding human dermal fibroblasts (hDF) on these nanofiber mats, the nanofiber mats with high chitin content, in particular, showed cell penetration and migration inside the scaffold; oppositely to pure PCL nanofiber mats, where the cells stayed on the surface. This finding is similar to those reported in other papers, where 3D structures and increased surface areas generally supported cell growth due to the improved possibility of cell infiltration and migration into the scaffold. The authors mentioned that for this effect, the pores in the electrospun nanofiber mats do not have to be larger than the cell diameters, but cells were found to perform amoeboid movements to migrate even through smaller pores by pushing the neighboring fibers away. Instead, hydrophilicity and biochemical signals were found to be more important to promote cell ingrowth than perfectly fitting pore sizes.
Min et al. used an electrospinning method based on two separate syringes to prepare PLGA nanofibers with chitin electrosprayed nanoparticles
[59]. They seeded normal human oral keratinocytes and normal human epidermal keratinocytes on these nanofiber composites and found the PLGA/chitin nano-composite fibers to be well suitable for tissue engineering scaffolds, due to their biomimetic 3D structure, similar to the collagen/glycosaminoglycan composite structure in the extracellular matrix.
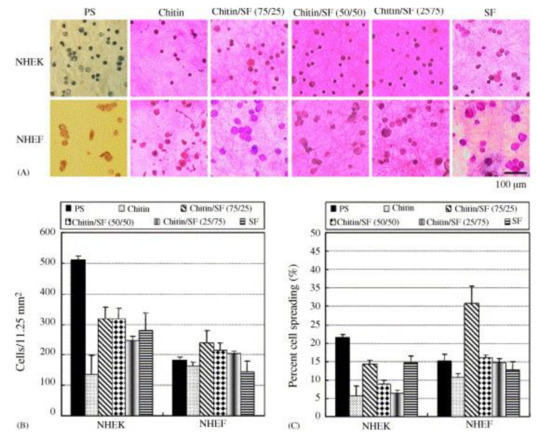
Another blend was investigated by Park et al., who used electrospinning to prepare chitin/silk fibroin (SF) blends from HFIP as solvent
[60]. Normal human epidermal keratinocytes (NHEK) and fibroblasts (NHEF) were seeded onto these nanofiber mats after crystallization of the SF fraction in water vapor. While for attachment of NHEK cells, the reference polystyrene substrate performed best, cell spreading of NHEF cells was superior on chitin/SF nanofiber mats, as visible in
Figure 3. This finding was explained by the high biocompatibility and other supportive properties of chitin and SF.
Figure 3. (
A) Photographs and (
B) numbers of NHEK and NHEK adhered on the substrates; (
C) percentage of cell spreading (
n = 4). Reprinted from
[60], with permission from Elsevier.
As mentioned before, chitosan combines many of the positive physical and chemical properties of chitin with water-solubility, making it more easily electrospinnable. This is why diverse groups investigated electrospun chitosan or chitosan blend fibers. A recent review of electrospinning chitosan-based solutions for tissue engineering was given by Qasim et al.
[61]. Some exemplary blend partners for chitosan are poly(ethylene oxide) PEO
[62][63], collagen
[64], PVA
[65][66], PCL
[67], and hydroxyapatite
[68].
After this brief overview of materials correlated to the extracellular matrix, the next sections - which can be found in the original paper - will show progress in using electrospun man-made polymers as scaffolds in tissue engineering.