The lack of specific treatment for chikungunya fever makes the need for anti-chikungunya virus agents more crucial. This study was conducted to evaluate 132 extracts obtained by sequential solvent extraction from 21 medicinal plants for cytopathic effect inhibitory activity using virus-infected Vero cells in two different sample introduction modes. Among the extracts, 42 extracts (31.8%) from 12 plants in the concurrent mode and three extracts (2.3%) from a plant in the non-concurrent mode displayed strong cytopathic effect inhibitory activity (cell viability ≥70%). Viral load quantification analysis unveiled that the extracts of Clinacanthus nutans (chloroform, ethyl acetate, and ethanol), Hydrocotyle sibthorpioides (ethanol), and Ocimum americanum (ethanol and methanol) hindered the release of viral progeny from the infected cells while the extracts of Ficus deltoidea (ethanol), Gynura bicolor (water), H. sibthorpioides (water), and O. americanum (chloroform and ethyl acetate) blocked the entry of virus into the cells. The extracts of Diodella sarmentosa (ethyl acetate), Diplazium esculentum (chloroform, ethyl acetate, and ethanol), and G. bicolor (ethanol) possessed virucidal effect and caused 5.41-log to 6.63-log reductions of viral load compared to the virus control. The results indicate that these medicinal plants are potential sources of anti-chikungunya virus agents that have varied modes of action.
1. Introduction
Chikungunya virus is an enveloped, single-stranded, positive-sense RNA virus belonging to the genus
Alphavirus of the
Togaviridae family. It is an arthropod-borne virus causing chikungunya fever in humans
[1]. The virus was first isolated from a febrile patient in the southern Tanzania in 1952–1953
[2]. Subsequent outbreaks of chikungunya infection have largely been confined to the countries in sub-Saharan Africa and Asia
[3]. However, over the last two decades, the virus has caused devastating epidemics in India, Southeast Asia and Pacific Islands, and islands in the Indian Ocean, leading to over six million cases of infection
[4]. Since 2013, the virus has spread and established its autochthonous transmission in the Western Hemisphere, resulting in over two million suspected cases being reported in almost 50 countries in the Americas. The virus has been documented in 114 countries and territories
[5]. Recent analysis indicates that this virus caused an average yearly loss of over 106,000 disability-adjusted life years for the period 2010–2019
[6]. According to the surveillance done by the European Centre for Disease Prevention and Control, at least 170,000 cases of chikungunya fever occurred globally in the year 2020
[7]. Malaysia recorded 2556 cases in the same year and about 86% of the cases happened in the states of Perak and Penang
[8].
Chikungunya virus is mainly transmitted via a bite by the infected mosquitoes
Aedes aegypti and
Aedes albopictus. The virus can cross the placenta and goes into the fetus in the vertical transmission mode, which results in higher rates of infant morbidity
[9]. Upon an acute infection, 80–97% of patients are symptomatic
[10] and their clinical manifestations include arthralgia or polyarthralgia, high fever, headache, myalgia, skin rashes, joint swelling, and nausea
[11]. Although the mortality rate is relatively low (0.07%), polyarthralgia can persist in the patients for several months or even years after resolution of the acute phase of infection, this being the most common long-term sequel of chikungunya virus infection
[6][12]. Presently, patients with chikungunya fever are treated with antipyretic, analgesic, or anti-inflammatory drugs for symptomatic relief
[5]. While efforts have been pursued to develop safe and effective vaccines for prophylaxis and antiviral drugs for therapeutics
[13][14][15], currently there is no licensed vaccine or drug available against chikungunya virus. This emphasizes the need for more antiviral drugs.
According to the World Health Organization estimates, approximately 80% of the world population use medicinal plants for some aspects of primary health care
[16]. Plants are able to produce arrays of phytochemicals with diverse chemical structures such as alkaloids, terpenoids, essential oils, flavonoids, and polyphenols. Many of these phytochemicals, which are derived from the different pathways of secondary metabolism, serve as chemical weapons for the plants against microbial infections, predations by insects and herbivores. They may also be indicators of environmental stress
[17]. These phytochemicals are also found to have various biological activities which are beneficial to human health. The antiviral activities of medicinal plants have been well documented against human immunodeficiency
[18][19], influenza, herpes simplex
[20][21], hepatitis
[21][22], and dengue viruses
[23][24]. A total of 17 extracts are reported to have anti-chikungunya virus activity from the screenings of 84 medicinal or endemic plants
[25][26]. Epigallocatechin gallate derived from
Camellia sinensis and curcumin from
Curcuma longa are reported to prevent chikungunya virus from attachment to cells
[27][28] while harringtonine from
Cephalotaxus harringtonia is able to block the replication of the virus in vitro
[29].
Twenty-one species of medicinal plants belonging to 19 families were selected for the present study and extracted sequentially using six solvents of increasing polarity. The medicinal or folkloric uses of these medicinal plants are shown in
Table 1. The phytochemicals are segregated into different extractants based on their polarity and solubility during sequential solvent extraction
[30]. Less polar solvents such as hexane and chloroform could extract alkaloids, coumarins, fatty acids, and terpenoids while more polar solvents such as ethyl acetate, ethanol, methanol, and water could yield saponins, tannins, flavones, polyphenols, terpenoids, anthocyanins, polypeptides, and lectins from plants
[31]. The objectives of this study were to evaluate the plant extracts for cytopathic effect inhibitory activity using chikungunya virus-infected African monkey kidney epithelial (Vero) cells in two different sample introduction modes, i.e., concurrent and non-concurrent modes. In the concurrent mode, the plant extracts and the virus inoculum were introduced simultaneously to the cells whereas for the non-concurrent mode, the cells were incubated with the extracts for 24 h before the addition of the virus inoculum. The modes of action of the selected active extracts were assessed based on the quantification of viral load using real-time reverse-transcriptase polymerase chain reaction (RT-PCR). The results of this study highlighted that medicinal plant extracts possess anti-chikungunya virus activity with varied modes of action.
Table 1. Details and uses of selected medicinal plants.
| Plant Name |
Family |
Vernacular Name |
Part Used |
Medicinal or Folkloric Uses |
Voucher Number |
| Ailanthus triphysa (Dennst.) Alston |
Simaroubaceae |
White siris |
Leaf |
Hypertension, bronchitis, dysentery [32] |
UTAR/FSC/11/004 |
| Archidendron jiringa (Jack) I.C.Nielsen |
Leguminosae |
Djengkol bean |
Seed |
Bladder stones, hypertension, diabetes [33] |
Nil |
| Arundina graminifolia (D.Don) Hochr. |
Orchidaceae |
Grass orchid |
Leaf |
Snake bites, rheumatism, stomachache [34] |
UTAR/FSC/10/011 |
| Azadirachta indica A.Juss. |
Meliaceae |
Neem |
Leaf |
Leprosy, skin ulcers, biliousness, epistaxis, eye problem, anorexia, intestinal worms [35] |
UTAR/FSC/11/001 |
| Basella alba L. |
Basellaceae |
Ceylon spinach, Malabar spinach |
Leaf |
Constipation, liver and urinary diseases, catarrh, gonorrhea, boils, sore throat, hypertension, burns [36] |
UTAR/FSC/10/014 |
| Beta vulgaris L. |
Amaranthaceae |
Beetroot |
Root |
Dandruff, decreased libido, constipation, joint pain [37] |
Nil |
| Clinacanthus nutans (Burm.f.) Lindau |
Acanthaceae |
Sabah snake grass |
Leaf |
Diabetes, dysentery, eye diseases, skin rashes, allergic responses, insect and snake bites [38] |
UTAR/FSC/11/003 |
| Curcuma longa L. |
Zingiberaceae |
Turmeric |
Rhizome |
Stomachic and intestinal diseases, arthritis, gall stones, emmenagogue, bruise, as a tonic [39] |
Nil |
| Diodella sarmentosa (Sw.) Bacigalupo & Cabral ex Borhidi |
Rubiaceae |
Tropical buttonweed |
Leaf and stem |
Ulcers, snake bite, rheumatic inflammatory disorders, venereal diseases [40] |
UTAR/FSC/10/018 |
| Diplazium esculentum (Retz.) Sw. |
Athyriaceae |
Vegetable fern |
Leaf and stem |
Constipation, hypertension [41] |
UTAR/FSC/10/023 |
| Ficus deltoidea Jack |
Moraceae |
Mistletoe fig |
Leaf |
Wounds, rheumatism, sores, as an after-birth tonic [42] |
UTAR/FSC/10/021 |
| Gynura bicolor (Roxb. ex Willd.) DC. |
Compositae |
Okinawa spinach |
Leaf |
Blood circulation improvement, diabetes, dysmenorrhea, hemoptysis, post-labor recovery [43] |
UTAR/FSC/11/005 |
| Homalocladium platycladum (F.Muell.) L.H.Bailey. |
Polygonaceae |
Centipede plant |
Stem |
Skin swelling, sores, insect and snake bites, fracture injuries, fever [44] |
UTAR/FSC/10/017 |
| Hydrocotyle sibthorpioides Lam. |
Araliaceae |
Lawn marsh pennywort |
Whole plant |
Cough, cold, fever, zoster, eczema, hepatitis, jaundice [45] |
UTAR/FSC/10/019 |
| Manilkara zapota (L.) P.Royen |
Sapotaceae |
Sapodilla, Ciku |
Fruit |
Diarrhea, pulmonary complaints [46] |
Nil |
| Ocimum americanum L. |
Lamiaceae |
Hoary basil |
Leaf |
Fever, colds, dysentery, toothache, migraine [47] |
UTAR/FSC/10/013 |
| Parkia speciosa Hassk. |
Leguminosae |
Stink bean |
Seed and pod |
Urinary infections, diabetes, loss of appetite [48] |
UTAR/FSC/10/015 |
| Petroselinum crispum (Mill.) Fuss |
Apiaceae |
Parsley |
Leaf and stem |
Skin diseases, eczema, hypertension, diabetes, nosebleed, constipation pain, baldness [49] |
UTAR/FSC/10/024 |
| Salacca zalacca (Gaertn.) Voss |
Arecaceae |
Salak |
Fruit |
Diabetes [50] |
Nil |
| Sechium edule (Jacq.) Sw. |
Cucurbitaceae |
Chayote |
Leaf and stem |
Kidney stones, hypertension [51] |
UTAR/FSC/10/022 |
| Strobilanthes crispus (L.) Blume |
Acanthaceae |
Yellow strobilanthus, “kejibeling” |
Leaf |
Kidney stones, enhance immune system [52] |
UTAR/FSC/10/020 |
2. Results and Discussion
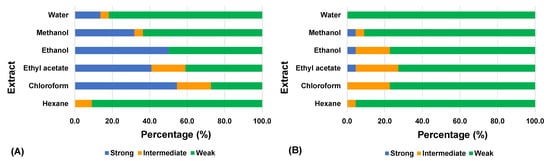
A total of 132 extracts obtained from 21 plant species were subjected to the antiviral activity screening against the chikungunya virus. As six different solvents were used, each extractant yielded 22 extracts. The ability of an extract to protect Vero cells from the cytopathic effect caused by the virus was used as a measurement of antiviral activity for the extract. As an extract is a mixture of many phytochemicals extracted from a particular plant part, it may contain compounds that are toxic to Vero cells. Thus, it is necessary to determine the non-toxic concentrations for use in the cytopathic effect inhibitory assay. As such, a standardized test concentration range of an extract is not feasible.
In order to express and classify the cytopathic effect inhibitory activity of an extract, three scales were established based on percentage of cell viability, these being strong inhibitory activity when the cell viability is ≥70%, intermediate inhibitory activity when the cell viability is 31–69%, and weak inhibitory activity when the cell viability is ≤30%. The inhibitory activity for each extract is shown in Table 2. Forty-two extracts (31.8%) were found to have strong inhibitory activity in the concurrent mode compared to only three extracts (2.3%) in the non-concurrent mode. These extracts were derived from 12 medicinal plants, i.e., Azadirachta indica, Clinacanthus nutans, Diodella sarmentosa, Diplazium esculentum, Ficus deltoidea, Gynura bicolor, Hydrocotyle sibthorpioides, Homalocladium platycladum, Ocimum americanum, Petroselinum crispum, Sechium edule, and Strobilanthes crispus. The results indicate that the cytopathic effect inhibitory activity was dependent on plant species and sample introduction mode. The results also suggest that phytochemicals in the extracts could exert an inhibitory effect against the virus in the concurrent mode but lost their activity in the non-concurrent mode. The exposure of Vero cells to the extracts for 24 h before the addition of virus inoculum could result in the metabolism of active phytochemicals into metabolites devoid of inhibitory activity. An exception was noted for the three extracts with strong inhibitory activity in the non-concurrent mode. They were ethyl acetate, ethanol, and methanol extracts of F. deltoidea. The corresponding cell viabilities in the concurrent mode were 66.8% ± 4.2% at 10 µg/mL, 71.9% ± 5.3% at 40 µg/mL, and 1.5% ± 2.9% at 40 µg/mL, respectively, and increased to 76.5% ± 4.1% (p = 0.046), 90.3% ± 0.8% (p = 0.024), and 79.8% ± 6.7% (p < 0.001), respectively, in the non-concurrent mode, suggesting that the metabolites produced (in the non-concurrent mode) may have stronger activity than their parent compounds.
Table 2. Classification of cytopathic effect inhibitory activity of each medicinal plant extract for the concurrent and non-concurrent modes.
| Plant |
Part |
Concurrent Mode |
Non-Concurrent Mode |
| |
Extract # |
HX |
CF |
EA |
EN |
MN |
WT |
HX |
CF |
EA |
EN |
MN |
WT |
| Ailanthus triphysa |
Leaf |
W |
W |
W |
W |
W |
W |
W |
W |
W |
W |
W |
W |
| Archidendron jiringa |
Seed |
W |
I |
W |
W |
W |
W |
W |
I |
W |
W |
W |
W |
| Arundina graminifolia |
Leaf |
W |
W |
W |
W |
W |
W |
W |
I |
I |
W |
W |
W |
| Azadirachta indica |
Leaf |
I |
S |
S |
S |
S |
W |
W |
W |
W |
W |
W |
W |
| Basella alba |
Leaf |
W |
I |
W |
W |
W |
W |
I |
I |
I |
W |
W |
W |
| Beta vulgaris |
Root |
W |
W |
W |
W |
W |
W |
W |
W |
W |
W |
W |
W |
| Clinacanthus nutans |
Leaf |
W |
S |
S |
S |
S |
W |
W |
W |
W |
W |
I |
W |
| Curcuma longa |
Rhizome |
W |
I |
W |
W |
W |
I |
W |
W |
W |
W |
W |
W |
| Diodella sarmentosa |
Leaf and stem |
W |
S |
S |
S |
W |
W |
W |
I |
W |
I |
W |
W |
| Diplazium esculentum |
Leaf and stem |
W |
S |
S |
S |
S |
W |
W |
W |
W |
I |
W |
W |
| Ficus deltoidea |
Leaf |
W |
S |
I |
S |
W |
W |
W |
I |
S |
S |
S |
W |
| Gynura bicolor |
Leaf |
W |
S |
S |
S |
S |
S |
W |
W |
I |
W |
W |
W |
| Homalocladium platycladum |
Stem |
W |
S |
S |
S |
I |
S |
W |
W |
I |
W |
W |
W |
| Hydrocotyle sibthorpioides |
Whole plant |
W |
S |
I |
W |
S |
W |
W |
W |
W |
W |
W |
W |
| Manilkara zapota |
Fruit |
W |
W |
W |
W |
W |
W |
W |
W |
W |
W |
W |
W |
| Ocimum americanum |
Leaf |
I |
S |
S |
S |
S |
S |
W |
W |
W |
W |
W |
W |
| Parkia speciosa |
Pod |
W |
I |
I |
W |
W |
W |
W |
W |
W |
W |
W |
W |
| Parkia speciosa |
Seed |
W |
W |
W |
W |
W |
W |
W |
W |
W |
W |
W |
W |
| Petroselinum crispum |
Leaf and stem |
W |
S |
S |
S |
W |
W |
W |
W |
W |
I |
W |
W |
| Salacca zalacca |
Fruit |
W |
W |
W |
W |
W |
W |
W |
W |
W |
W |
W |
W |
| Sechium edule |
Leaf and stem |
W |
S |
S |
S |
W |
W |
W |
W |
I |
I |
W |
W |
| Strobilanthes crispus |
Leaf |
W |
S |
I |
S |
S |
W |
W |
W |
W |
W |
W |
W |
The data in
Figure 1 indicate that extractants such as chloroform, ethyl acetate, ethanol, and methanol resulted in higher activity compared to hexane and water, both in the concurrent mode and non-concurrent mode. The type of solvent used to extract phytochemicals from plants is an important contributing factor to the results of the bioassay. Phytochemicals of a plant part are solubilized in an extractant based on their polarity
[30].
Figure 1. Classification of medicinal plant extracts according to their cytopathic effect inhibitory activity against chikungunya virus in (A) concurrent mode and (B) non-concurrent mode. The number of extracts for each extractant is 22. The inhibitory activity is measured based on the percentage of viable cells protected by an extract from the cytopathic effect caused by the virus. Strong: cell viability ≥70%; intermediate: cell viability 31–69%; weak: cell viability ≤30%.
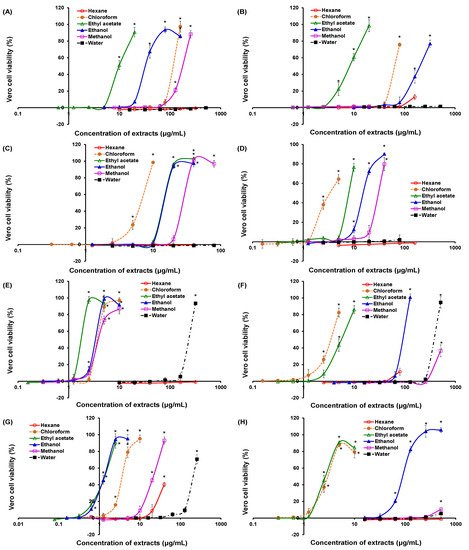
Among the 45 extracts which exhibited strong inhibitory activity, 20 extracts of seven plants from the concurrent mode and one extract of a plant from the non-concurrent mode were able to protect ≥90% of Vero cells from the cytopathic effect caused by the virus, as shown in Figure 2. These 21 extracts are of great potential for further drug developments. A wide half-maximal effective concentration (EC50) range was observed for these extracts, ranging from 1.33 µg/mL for the ethanol extract of O. americanum to 394.0 µg/mL for the water extract of H. sibthorpioides (Table 3). Consequently, the selectivity indices for these extracts ranged from 2.62 to 170.2. The indices for the ethanol extract of S. edule and the water extract of H. sibthorpioides could not be calculated as no significant cytotoxicity (p > 0.05) was recorded.
Figure 2. Viability of Vero cells infected by chikungunya virus and treated with different extracts of medicinal plants. (A) Clinacanthus nutans; (B) Diodella sarmentosa; (C) Diplazium esculentum; (D) Ficus deltoidea; (E) Gynura bicolor; (F) Hydrocotyle sibthorpioides; (G) Ocimum americanum; (H) Sechium edule. All plants are shown for the concurrent mode except Ficus deltoidea, which is in the non-concurrent mode. The cell viability is measured using the neutral red uptake assay. The notated asterisks (*) denote significant differences (p < 0.05) among concentrations within an extract by one-way ANOVA.
Table 3. Selectivity indices and viral RNA copy numbers for the selected active medicinal plant extracts against chikungunya virus.
| Plant |
Extract |
Half-Maximal Cytotoxic Concentration, CC50 (µg/mL) |
Mode ^ |
Half-Maximal Effective Concentration, EC50 (µg/mL) |
Selectivity Index (= CC50/EC50) |
Viral RNA Copy Number (Molecules/µL) |
Log Reduction # |
| Clinacanthus nutans |
Chloroform |
602.67 ± 9.29 |
C |
120.67 ± 4.62 |
4.99 |
7.75 × 109 ± 1.69 × 109 * |
0.89 |
| Ethyl acetate |
133.00 ± 9.17 |
C |
9.93 ± 0.91 |
13.39 |
1.68 × 1010 ± 0.51 × 1010 * |
0.55 |
| Ethanol |
>640 |
C |
31.30 ± 0.95 |
>20.45 |
8.72 × 109 ± 1.25 × 109 * |
0.83 |
| Diodella sarmentosa |
Ethyl acetate |
203.33 ± 6.11 |
C |
8.33 ± 0.57 |
24.40 |
1.83 × 105 ± 1.07 × 105 * † |
5.51 |
| Diplazium esculentum |
Chloroform |
99.00 ± 3.61 |
C |
6.80 ± 0.26 |
14.56 |
4.23 × 104 ± 0.59 × 104 * † |
6.15 |
| Ethyl acetate |
184.33 ± 9.24 |
C |
14.07 ± 0.06 |
13.10 |
1.38 × 104 ± 0.62 × 104 * † |
6.63 |
| Ethanol |
220.67 ± 1.53 |
C |
14.30 ± 0.20 |
15.43 |
3.40 × 104 ± 1.02 × 104 * † |
6.24 |
| Methanol |
461.00 ± 1.73 |
C |
29.70 ± 0.60 |
15.52 |
1.12 × 109 ± 0.11 × 109 * † |
1.73 |
| Ficus deltoidea |
Ethanol |
>640 |
NC |
15.20 ± 0.20 |
>42.10 |
4.42 × 106 ± 2.71 × 106 † |
4.13 |
| Gynura bicolor |
Chloroform |
117.67 ± 9.50 |
C |
3.65 ± 0.06 |
32.21 |
3.50 × 109 ± 1.18 × 109 * † |
1.23 |
| Ethyl acetate |
31.33 ± 4.16 |
C |
1.91 ± 0.03 |
16.43 |
3.71 × 108 ± 2.90 × 108 † |
2.21 |
| Ethanol |
55.00 ± 3.46 |
C |
3.62 ± 0.10 |
15.18 |
2.33 × 105 ± 0.58 × 105 * † |
5.41 |
| Water |
> 640 |
C |
244.67 ± 4.73 |
>2.62 |
3.29 × 105 ± 1.78 × 105 † |
5.26 |
| Hydrocotyle sibthorpioides |
Ethanol |
610.33 ± 9.50 |
C |
95.33 ± 2.47 |
6.40 |
4.01 × 1010 ± 1.54 × 1010 * |
0.17 |
| Water |
- |
C |
394.00 ± 6.93 |
- |
4.39 × 105 ± 2.74 × 105 † |
5.13 |
| Ocimum americanum |
Chloroform |
86.33 ± 4.73 |
C |
3.61 ± 0.11 |
23.92 |
5.50 × 105 ± 0.75 × 105 † |
5.03 |
| Ethyl acetate |
60.83 ± 2.02 |
C |
1.37 ± 0.06 |
4.45 |
3.57 × 105 ± 0.26 × 105 † |
5.22 |
| Ethanol |
226.33 ± 9.87 |
C |
1.33 ± 0.10 |
170.18 |
1.71 × 1010 ± 0.48 × 1010 * |
0.54 |
| Methanol |
>640 |
C |
21.93 ± 0.84 |
>29.18 |
7.81 × 109 ± 2.32 × 109 * |
0.88 |
| Sechium edule |
Ethyl acetate |
100.67 ± 9.29 |
C |
2.71 ± 0.25 |
37.10 |
7.43 × 109 ± 2.79 × 109 * |
0.90 |
| Ethanol |
- |
C |
90.33 ± 0.28 |
- |
1.14 × 1010 ± 0.16 × 1010 * |
0.72 |
| Chloroquine |
- |
16.33 ± 0.76 |
NC |
9.05 ± 0.05 |
2.89 |
1.68 × 106 ± 0.49 × 106 * † |
4.55 |
| C |
1.92 ± 0.13 ** |
13.65 |
3.94 × 105 ± 0.70 × 105 † |
5.18 |
| Virus inoculum |
- |
- |
- |
- |
- |
6.25 × 105 ± 2.09 × 105 |
- |
| Virus control |
- |
- |
- |
- |
- |
5.96 × 1010 ± 3.33 × 1010 |
- |
In order to yield some indications of the antiviral mechanisms of the 21 extracts, quantification of the viral copy number in the experiments was performed using a real-time RT-PCR. The results are shown in Table 3. All extracts of C. nutans and S. edule, ethanol extract of H. sibthorpioides, and ethanol and methanol extracts of O. americanum produced a viral copy number similar to the virus control (p > 0.05), suggesting the virus was successfully replicated in the Vero cells but the release of the viral progeny was inhibited by these extracts, and this prevented the occurrence of cytopathic effect in the cells. In contrast, the viral copy numbers for the infected cells treated with the extracts of F. deltoidea (ethanol), G. bicolor (water), H. sibthorpioides (water), and O. americanum (chloroform and ethyl acetate) were not significantly different (p > 0.05) from the copy number of the viral inoculum. The results suggested that these extracts may work as a fusion inhibitor and block the entry of the virus into the cells. The virus was not able to replicate in the experiments and the copy number remained similar to that of the viral inoculum throughout the 72-h incubation period. The viral copy numbers for five extracts, i.e., ethyl acetate extract of D. sarmentosa, chloroform, ethyl acetate, and ethanol extracts of D. esculentum, and ethanol extract of G. bicolor, were significantly lower (p < 0.05) than that of the virus inoculum. These extracts caused 5.41-log to 6.63-log reductions of viral load compared to the virus control (Table 3) as quantified by real-time RT-PCR, suggesting the active phytochemicals in the extracts possessed a virucidal effect on the chikungunya virus. The viral copy number indicates that phytochemicals may have different modes of action against the virus, as illustrated by the extracts of H. sibthorpioides and O. americanum whereby these extracts could prevent the release of viral progeny and the entry of the virus into cells. Similarly, the extracts of G. bicolor could kill the virus, as well as block the virus from entry into the cells.
Chloroquine, which was used as a positive control, is reported to interfere with the protonation of the endocytic vesicles thereby raising the endosomal pH and preventing the fusion of chikungunya virus to the host cell
[53]. The EC
50 value of chloroquine obtained for the non-concurrent mode (9.05 µg/mL or 17.5 µM) was significantly higher (
p < 0.001) than the concurrent mode (1.92 µg/mL or 3.72 µM) (
Table 3), suggesting that the metabolism of chloroquine in the Vero cells may have reduced its efficacy by producing non-active metabolites or metabolites with reduced efficacy against the chikungunya virus. The EC
50 value (3.72 µM) obtained in this study was generally lower than the values (5.0–11 µM) reported in the literature, probably resulting from the types of cells and the virus strains used
[54][55][56].
To the best of our knowledge, this study constitutes the first report of the antiviral properties of the medicinal plants
D. sarmentosa,
D. esculentum,
F. deltoidea,
G. bicolor,
H. platycladum, and
S. edule.
Azadirachta indica, popularly known as neem, has been extensively used in the Unani, Ayurveda, and Chinese traditional systems of medicine
[35]. Raghavendhar et al. studied the water extract of the bark of
A. indica against chikungunya virus and reported that the extract did not reduce the plaque formation in Vero cells
[57]. In contrast, the current study shows that the chloroform, ethyl acetate, ethanol, and methanol extracts of the leaves of
A. indica had strong cytopathic effect inhibitory activity against the virus (
Table 2). For
C. nutans,
H. sibthorpioides,
O. americanum,
P. crispum, and
S. crispus, the results of this study further strengthen the case for these plants as potential sources of antiviral compounds. The antiviral activity of
C. nutans has well been documented against herpes simplex virus types 1 and 2
[58][59], human papillomavirus
[60], and dengue virus
[61]. The methanol extract and the asiaticoside isolated from
H. sibthorpioides possess anti-dengue virus activity
[62] and anti-hepatitis B virus activity
[63], respectively. The dichloromethane and methanol extracts of
O. americanum and the methanol extract of
S. crispus displayed anti-herpes simplex virus activities
[64][65]. The methanol extract of
P. crispum has been reported to have inhibitory activity against the Sindbis virus, which like the chikungunya virus is an alphavirus
[66]. Further studies need to be carried out to elucidate the identity of antiviral compounds in the active extracts, the inhibitory or virucidal potential for the isolated pure compounds, and the possible synergistic effects among the isolated compounds in targeting different mechanisms of the life cycle of chikungunya virus.
3. Conclusions
For this research 132 extracts from 21 medicinal plant species were evaluated for cytopathic effect inhibitory activity against the chikungunya virus using concurrent and non-concurrent sample introduction modes. The inhibitory effect of the extracts was dependent on plant species, extract concentration, type of extractant, and sample introduction mode. More extracts were found to have strong inhibitory activity in the concurrent mode than in the non-concurrent mode. Analysis of selected 21 extracts from eight plants with a strong inhibitory activity using real-time RT-PCR indicates that the active extracts targeted the chikungunya virus life cycle at different stages, including inhibition of virus entry into Vero cells, blocking the release of viral progeny from the cells, and virucidal effect on the virus. Some of the medicinal plants such as G. bicolor, H. sibthorpioides, and O. americanum even possessed multiple antiviral mechanisms. The bioactive compounds in the plant extracts could be isolated and characterized as lead compounds for potential pharmaceutical developments into anti-chikungunya virus drugs. The plant extracts could also be evaluated against other viruses such as dengue virus, which causes another endemic mosquito-borne disease in Malaysia. The results of this study reiterated the fact that medicinal plant extracts contain many phytochemicals with biological activities. Medicinal plants could be explored as an accessible and sustainable source of chemotherapeutic agents for the treatment of emerging or re-emerging viral diseases. More collaborative efforts are needed to pursue the exploration of medicinal plants for human health benefits.