1000/1000
Hot
Most Recent

Antimicrobial Peptides can be defined as the molecules of the innate immune system present in all life forms, ranging from bacteria to human beings. The innate immune system is a defence system working non-specifically against injury or infection in the barrier surface. AMPs are composed of a sequence of amino acid ranging from 5 to 50 chains, usually L-amino acids.
Most of the AMPs are positively charged peptides (due to a high content of arginine and lysine residues) that are capable of destroying pathogens directly. The benefit of using peptides as antimicrobial agents is that it shows effectiveness against Gram-positive and Gram-negative bacteria, including multidrug-resistant bacteria [1][2]. Most of the AMPs function by disrupting the physical structure of the microbial membrane and/or by targeting intracellular bodies [3]. AMPs have proven to have the ability to fight against various health issues due to the presence of immunomodulatory properties [4]. AMPs are effective in preventing skin cancer, treating wound infection and limiting autoimmune diabetes [5][6][7]. Promising results of AMPs have suggested its use in novel therapeutic drugs [3].
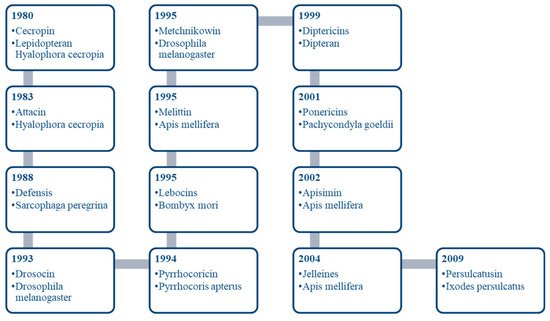
AMPs can be synthesised from animals, plants, protozoa, fungi and bacteria. Among all the species, insects are found to be the largest living entity of AMPs because of their excellent ability to tolerate changes and their resistant property towards pathogens [8]. Insects are considered an excellent source of AMPs because of their huge biodiversity [9]. The characterisation of the innate immune system of insects depends on cellular immunity, which includes phagocytosis, and humoral immunity, which includes the excretion of proteins and peptides [10]. The way an insect responds to fight infections can be categorised in two categories: The constitutive response, which is perpetually ready to fight, and the induced response, which takes 1–3 h to generate [11]. The first insect AMP derived was Cecropin from lepidopteran Hyalophora cecropia [12][13]. More than 3000 AMPs are known till now, among which 2301 are isolated from animals. Hermetia illucens (the black soldier fly) is found to be the most propitious source due to its resisting ability against a huge range of pathogens [14]. Hermetia illucens has a strong immune system as it feeds on decaying organism and manure, which contains high amount of pathogens. Figure 1 represents the history of antimicrobial peptides derived from insects.
In recent years, AMPs have gained a huge interest in various fields due to their low toxicity and ability to fight a wide spectrum of pathogenic microbes. One is food packaging, involving studies based on the incorporation of AMPs into packaging material that will be in direct contact with food or interact with the headspace to resist microbial contamination in food [15]. The advantages of using antimicrobial peptides in food packaging over antimicrobial agents [16]: Improves food safety by preventing the development of resistant strains of microorganisms; Prohibited use of some of the antimicrobial agents due to toxicological reasons; Distinctive mode of action of antimicrobial peptides with effective results.
The antimicrobial activity of AMP depends on peptide length, charge and hydrophobic nature, and this leads into grouping of AMPs based on some dissimilarities in attributes. Based on the structure, AMPs can be categorised into four types: α helical peptide, cysteine-rich peptide, proline-rich peptide and glycine-rich peptide [17]. Some studies have also discussed classification into α helical peptide and β sheet peptide. In α helical peptides, cysteine is present, which forms an intracellular disulphide bridge, and in β sheet peptides, a disulphide bond is found, which is useful in stabilizing the structure as well as crossing the cell membrane [18][19]. According to [20], AMPs can be classified based on the electrostatic charge into cationic (which have positive charge) and non-cationic (consists of negative charge) peptides. Based on the mode of action, peptides are divided into two categories: the membranolytic mechanism and non-membranolytic mechanism [21][22]. AMPs derived from insects are divided into three types: Defensins, Cecropins and peptides with an overrepresentation of Proline and/or Glycine residues [23]. In a study 50 genes encoding putative AMPs were identified from Hermetia Illucens, among which 6 attacins, 26 defensins, 7 cecropins, 10 diptericins and 4 knottin-like peptides were recorded [24]. Table 1 includes the classification of antimicrobial peptides.
| S.No. | Criteria for Classification | Peptides | Description | Examples | References |
|---|---|---|---|---|---|
| 1. | Structure | α helical peptide | Intramolecular disulphide bridge is formed by the cysteine | Cecropins | [18] |
| Cysteine rich peptide | Peptides with cysteine residues | Defensis | [9] | ||
| Glycine rich peptide | Consists of 14% to 22% glycine residues | Attacins | [25] | ||
| Proline rich peptide | Composed of 14–39 amino acids and contains proline residues | Drosocins | [9] | ||
| β sheet peptide | Consist of a disulphide bond, which helps in stabilizing the conformation | Defensis | [26] | ||
| 2. | Mode of action | Membranolytic | These peptides enter the microbial cell wall by disruption | Scolopendin 2 | [27] |
| Non- membranolytic | Peptide that enters the cell by endocytosis | Scolopendin 1 | [27] | ||
| 3. | Electrostatic charge | Cationic | Peptide with positive charge | Cecropins | [28] |
| Non- cationic | Peptides with negative charge and isolated from mammalian epithelia | Enkelytin | [28] |
AMPs are found to be useful in various fields of interest, which has led to the study of different methods for the efficient synthesis of peptides from the source. At present, there are three methods of synthesising peptides, which are chemical synthesis, enzymatic synthesis and synthesis using recombinant DNA technology. A brief comparison between all the three mechanism is discussed in the Table 2 .
| S.No. | Type of Synthesis | Advantages | Challenges | References |
|---|---|---|---|---|
| 1. | Chemical | Easy separation from side products and impure compounds. | Toxic byproducts and low yields. | [29][30] |
| 2. | Enzymatic | Helpful in synthesis of short chain peptides. It also has good stereo selectivity. | It becomes challenging while synthesis of long chain peptides. Low productivity and high cost of catalyst. | [30][31] |
| 3. | Recombinant DNA Technology | Convenient for large scale production. | Takes more time due to lengthy process. | [32][33] |
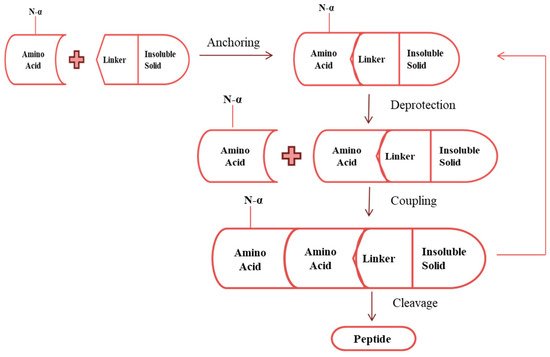
The synthesis of peptide using chemical reagents to conciliate peptide bonding was performed for the first time 50 years ago. Chemical synthesis of AMPs can be performed in two ways: synthesis in a solution and solid-phase peptide synthesis (SPPS). Synthesis in a solution is implemented by dissolving all the components in the solution [34]. While in SPPS, an insoluble solid is attached to a N-α-derivative of an amino acid through a linker. Then the protecting group (N-α group) is removed, followed by washing the complex with the solvent. Coupling of the second amino acid (containing N-α group) to the complex, either in the presence of activator or as a preactivated species, takes place. A solvent is used to wash the oligopeptide–linker–support complex, and unreacted matter is discarded. Repetition of withdrawing the protection group and the coupling cycle is performed until the required sequence of amino acids is achieved. Using the cleavage agent, peptide is generated in the form of free acid or amide ( Figure 2 ) [35].
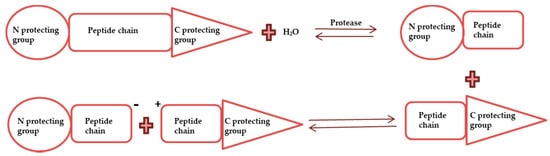
Enzymatic synthesis of peptides uses enzymes, such as pepsin, papain, trypsin and others, to conciliate a peptide bond. Enzymatic synthesis is better than chemical synthesis as it can be used to manufacture small peptides (2–5 oligomers), but it has a drawback that it is ineffective in the synthesis of long sequences [36]. It can be implemented in two ways: (i) reverse hydrolysis, which involves reversibility principle under the conditions that equilibrium is deliberately shifted towards peptide formation ( Figure 3 ), and (ii) transpeptidation, which involves breaking the peptide bond and forming an active acyl-enzyme intermediate that further results in peptide formation in the presence of nucleophile ( Figure 3 ) [31][37][38].
This mechanism involves cloning and gene expression, which produces a recombinant peptide, and E.coli is considered the most common host [39]. Synthesis of peptides using recombinant technology is considered the most economical method for massive production [40].
Hermetia illucens are present ubiquitously, and since it survives in highly contaminated environment, it is capable of producing a huge spectrum of antimicrobial peptides. H. illucens consume cheaper feed and also grow faster, which is a highly recommended attribute for AMP isolation [41][42]. Defensins, cecropin, attacin and lysosymes are considered as representative antimicrobial peptides of H. illucens [9]. The mechanism of harvesting AMP from insects involves some important fundamentals, as discussed below. General steps in AMP harvesting are mentioned in the flow chart ( Figure 4 ).
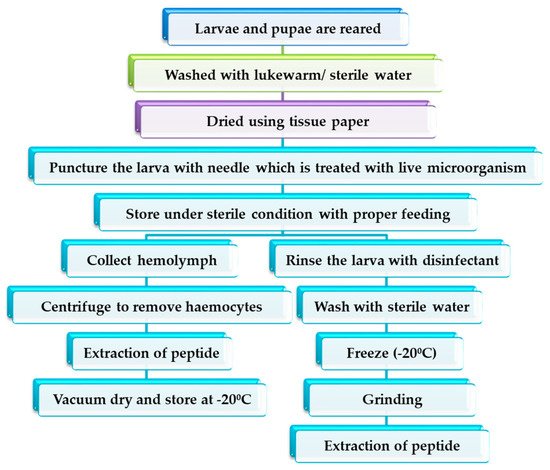
In the process of insect formation from larva, several growth and development stages occur, which are usually known as instar. There is no specification for considering any specific instar larva for the AMP collection. However, in a study, the lowest transcription level was observed during first-instar larval stages and the highest transcription level during the fifth-instar larval stage [43]. At the fifth stage, growth is completed, and metamorphosis is initiated [44]. Therefore, considering fifth-instar larva will be beneficial in a higher rate of synthesis of peptides. If the larva is to be used as a feed, prepupa is better than larva because of its high chitin content [45]. Rearing of larva varies from study to study, depending on the criteria of the research. In an investigation, researchers observed that aqueous extracts of black soldier fly larvae fed a cellulose diet and protein-rich diet showed antimicrobial activity against Gram-negative bacteria, while larvae fed chitin, cellulose, bacteria and plant oil had higher inhibitory activity against Gram-positive bacteria [24]. Chloroform extract of larva showed the highest inhibitory activity when fed lignin, bacteria and plant oil diets. In contrast, in a report by [46], larvae were starved after inoculating the Lactobacillus sp . to study the antimicrobial activity of H. illucens extract. At the industrial level, cottonseed press cake is a good choice as feed for H. illucens due to its sustainability and cheaper cost [47].
Microorganisms are injected into the body of a larva or haemolymph using a needle. Inoculated microorganisms should be in the stationary phase because natural microbial infection in insects occurs in the stationary phase [11]. The microorganism is injected either to isolate and analyse the characteristics of the AMP formed for that specific microorganism or to examine the influence of immunisation on the antimicrobial activity towards different microorganisms. Several reports have claimed the antimicrobial activity of AMPs harvested from H. illucens, among which a few are mentioned in Table 3 . In a study, AMPs from H. illucens were purified and investigated. AMPs were harvested from three samples: larvae that were immunised during the last instar stage, unimmunised larvae and mutilated larvae. From all the samples, haemolymph was collected and tested against E.coli and methicillin-resistant Staphylococcus aureus. Among all the samples, larvae that were immunised during the last instar stage showed the highest antimicrobial activity, while the mutilated group showed the least [42]. Zdybicka-Barabas observed that M. luteus -immunised haemolymph of H. illucens larvae showed inhibitory activity against Gram-positive bacteria, whereas E.coli -immunised larvae showed activity against Gram-positive and Gram-negative bacteria. Additionally, it was concluded that synthesis of AMPs vary depending on the bacteria used for the immune challenge [48]. Usually, when AMPs are treated with enzymes, such as trypsin or chymotrypsin, it results in a loss of antimicrobial activity. However, when H. illucens are immunised with L. casei and given the same treatment, they remain unaffected [46].
| S.No. | Source | Peptide | Harvesting Technique | Microorganisms Inhibited | References |
|---|---|---|---|---|---|
| 1. | Haemolymph | Solid phase extraction | Helicobacter pylori | [49] | |
| 2. | Crushed larva | stomoxynZH1 | RNA extraction using Trizol | S. aureus, E. coli, Rhizoctonia solani and Sclerotinia sclerotiorum | [50] |
| 3. | Grounded larvae | Maceration | E. coli, P. fluorescens, M. luteus and B. subtilis | [23] | |
| 4. | Larvae | Directly used as feed for piglets | Lactobacilli, D-streptococci | [51] | |
| 5. | Lyophilized larvae | Homogenised and extracted with acidic methanol | Methicillin resistant Staphylococcus aureus | [52] | |
| 6. | Haemolymph | cecropin-like peptide 1 | Solid-phase extraction and reverse-phase chromatography | E. coli, Enterobacter aerogens and Pseudomonas areuginosa | [53] |
| 7. | Grounded larva | Maceration using methanol | Salmonella and E. coli | [54] | |
| 8. | Larvae whose digestive tract was removed. Followed by treating with liquid nitrogen | Hidefensin-1, Hidiptericin-1 and HiCG13551 | TRIeasy- RNA isolation kit | Streptococcus pneumonia, E. coli and Staphylococcus aureus | [55] |
For AMP extraction from larvae, either haemolymph is collected, or the whole larva body is utilised. Hetru and Bulet collected the haemolymph in a precooled tube consisting of protease and melanisation inhibitors. Additionally, for small-sized insects, they froze the insects using liquid nitrogen, followed by grinding. Then, the powder was placed in water acidified with trifluoroacetic acid embodied with protease and melanisation inhibitor, followed by centrifugation and filtration [56]. Similarly, in most of the research, it was found that haemolymph is collected in ice-cold tubes consisting of phenylthiourea crystals to avoid coagulation. After collection, haemolymph is centrifuged to remove haemocytes [42]. Tabunoki proposed a method for collecting haemolymph from larva using a collection tube. This tube was prepared by making a hole at the bottom of a 0.5-mL centrifuge tube and then placing this tube in a 1.5 mL tube [57]. Samples were collected from grounded larvae in a solution of methanol, water and acetic acid for characterisation. Additionally, the method to isolate RNA was considered as follows: dissect larvae in ice-cold PBS, soak foregut/ midgut, hindgut and salivary glands in lysis buffer, and then freeze [23]. Another protocol was reported in which larvae were ground and suspended in 20% acetic acid solution, followed by boiling and then centrifugation [46].