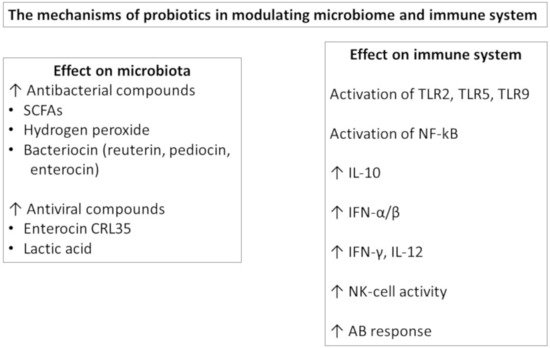
It has been recognized that microbiota plays a key role in shaping immune system maturation and activity. Since probiotic administration influences the microbiota composition and acts as a biological response modifier, the efficacy of an adjuvant for boosting vaccine-specific immunity is investigated.
1. Microbiome, Probiotics, Immune System and Vaccines
In recent years, much attention has been paid to the role of microbiota in shaping immune system development due to their impact on immune cell signaling and differentiation, since they are involved in driving both innate and acquired immunity
[1]. This role has also been demonstrated in the modulation of immune responses to vaccination
[2]. The microbiome can influence the microbial composition itself and the vaccine responses by different mechanisms: bacterial DNA, lipopolysaccharide (LPS) and flagellin can activate the Toll-Like-Receptor (TLR) pathways and, in turn, drive the dendritic cell (DC) responses. In mice, it has been shown that bacterial flagellin stimulated TLR5-mediated sensing that was necessary for antibody production to the influenza vaccine. Of note, TLR5-deficient mice, germ-free or antibiotic-treated animals had impaired responses to the influenza vaccine
[3][4]. Furthermore, a deficit or disruption in microbial communities, such as in antibiotic-induced dysbiosis, is able to reduce the vaccine responses particularly in early life. This was demonstrated in animal models, where dysbiosis reduced specific IgG responses to different vaccines; this effect was reversed by the fecal transfer of commensal microbiota
[5]. Notably, antibiotic administration did not alter the vaccine responses in adult mice, leading to the consideration that the infant microbiome is still developing and therefore characterized by low diversity, rapid change and high susceptibility
[3].
Since probiotic administration can influence the microbiota composition and diversity, the use of probiotics as supplements has been proposed for immunological outcomes, such as clinical improvements of the immune responses
[6]. It has been demonstrated that probiotics ensure, in a strain-specific way, immune modulation in viral infections with implications that are useful also when vaccine are considered. The concept that probiotics affect the vaccine response through modifications in the composition of the intestinal microbiota is supported by studies that verified changes in the stool microbiome after the intake of probiotics. Four small studies reported that stool microbiome changes, an abundance of particular strains and more bacterial diversity, induced by probiotic administration, were associated with greater responses to different vaccines
[7][8][9][10].
Understanding the mechanism behind probiotic action provides a rationale for the selection for probiotics
[11] (
Figure 1). At the gut level, probiotics are able to influence the gut barrier function by effector molecules such as protein HM0539 by LGG that promotes the expression of tight junction proteins
[12] or as conjugated linoleic acid (CLA) produced by several lactic acid bacteria that upregulate the expression of E-cadherin 1 and occludin in the gut
[13]. Orally administered probiotics may have direct effects by stimulating immunological receptors, such as TLRs, on the epithelial and immune cells and/or can secrete metabolites, such as the short chain fatty acids (SCFA), with immunomodulatory effects. Furthermore, they can be taken up by M cells, processed and then presented to the DCs. In this way, probiotics modulate DC polarization and function, influencing the subsequent T and B cell responses at the local and extraintestinal levels
[14]. The probiotic effects are more evident in the small intestine, where the number of endogenous bacteria is lower, but are also present in other body sites.
Figure 1. The mechanisms of probiotics in modulating.
In particular, probiotic bacteria can activate TLRs, leading to NF-kB and IRFs in immune cells. Lacticaseibacillus strains were able to prevent influenza A virus replication by activating type I IFN-dependent antiviral genes, and
L. paracasei CNCM-I-1518 modified pro- and anti-inflammatory cytokine release in the lungs after influenza infection
[15]. In mice, Bifidobacterium improved anti-influenza immune responses by decreasing IL-6 levels, also in the lungs, and higher IgG1 and IgG2 serum levels in probiotic-treated animals
[16]. The LPS of Gram-negative probiotics is a strong inducer of IL-10 in PBMC that contributes to the induction of IgA at the mucosal level
[17].
Regarding the mechanisms as adjuvant, Bron et al., reviewing the available data on the adjuvant activity of three strains of lactobacilli, according to the new taxonomic names of each strain/species of the Genus
Lactobacillus [18], emphasized the role of lipoteichoic acid (LTA) of the
Lactiplantibacillus plantarum (Lp.plantarum) strain, the secreted
Lacticaseibacillus rhamnosus (
Lc. rhamnosus) GG (also known as LGG) proteins p40 and p75, and the surface layer protein A (SlpA) of
Lactobacillus acidophilus (L. acidophilus) NCFM
[19]. These factors could represent the bacterial structural components supporting the vaccine adjuvant effect that has been shown since the pioneering papers from Link-Amster
[20]. These authors enrolled a cohort of 30 adults fed with a fermented milk containing, in addition to yogurt bacterial cultures, one strain of
L. acidophilus and one strain of
Bifidobacterium lactis, which showed beneficial effects during a course of vaccination against
Salmonella thyphi Ty21a. In particular, the treated group showed a 4.08-fold rise in specific IgA anti Ty2a LPS antibodies compared with a 2.48-fold rise in the control group
[20]. After that combination, in vitro and animal models as well as human studies have provided further support to the potential role of probiotic bacteria as adjuvants. Since data from laboratory studies are promising and represent the biologically plausible rationale for their use, novel evidence underlines the ability of probiotics to strength the efficacy of vaccination.
Therefore, probiotics are considered biological response modifiers, and among these effects, the efficacy of adjuvants for boosting vaccine-specific immunity has been highlighted
[21]. The effects of adjuvants on vaccination could be related to the increase in immunogenicity, the ability to influence the immunoglobulin response and the duration of immunity.
2. Human Studies
Despite the favorable results from in vitro and animal models, the literature available on human studies is not univocal and is often highly heterogeneous. In a recent systematic review, Zimmermann et al. showed that a beneficial effect was reported in half of the studies, with oral vaccination and parental influenza vaccination exerting the greater effect on vaccine response
[22].
The data regarding probiotic supplementation on influenza vaccine seroconversion in adults have been reported in a recent meta-analysis
[23]. Investigating the effect of probiotics on immune response to influenza vaccination in adults, the authors found a significant improvement in the H1N1, H3N2 and B strain serum-protection rate, which suggests that probiotics are effective in increasing immunogenicity by influencing seroconversion in adults vaccinated for influenza
[23]. Elderly subjects who were given a yogurt drink containing
L. casei had higher influenza-specific antibody titers
[24], and in healthy adults,
Limosilactobacillus fermentum (Ls. fermentum) improved influenza vaccine immunogenicity
[25]. In another study,
Lc. rhamnosus LGG administration ensured an increased protective rate for one strain but not for all strains present in that influenza vaccine in comparison to placebo
[26]. In a recent paper by Bianchini and colleagues, the effect of LGG administered for 3 months was tested on immune responses to an influenza vaccine in children and adolescents with type 1 diabetes. The results showed that the influenza vaccine was highly effective and safe in this population and that LGG administration did not modify the vaccine humoral responses versus the placebo-treated group. The LGG supplemented group presented reduced inflammatory responses from activated peripheral blood mononuclear cells (PBMCs), underlying the possible anti-inflammatory effects given by the systematic administration of the probiotics
[27].
Data regarding infants and children are more heterogeneous. Isolauri et al.
[28] demonstrated a significant effect of LGG on rotavirus IgA and IgM seroconversion after vaccination in infants of 2–5 months of age. The LGG supplementation was administered immediately before and for 5 days after the vaccine. In another study, Kukkonen et al. evaluated the effect of a mixture of four probiotics, given to the mothers from the 36th week and then to the newborns during the first 6 months of life
[29]. The infants were vaccinated with diphtheria, tetanus and pertussis at 3, 4 and 5 months and with anti-Hemophilus B (HiB) at 4 months. Antibody responses were evaluated at 6 months, showing a significantly increased seroconversion rate in response to vaccination for HiB but not for tetanus and diphtheria in the probiotic-treated group. For the latter, however, the probiotic supplementation did not negatively affect the vaccination outcomes, since the IgG titers were comparable between groups
[29].
In another study, Taylor and colleagues administered
Lc. LAVR1-A1 daily for the first 6 months of life. Then, the infant PBMCs were isolated and stimulated with various factors in vitro
[30]. The authors demonstrated that the administration of probiotics was accompanied by a reduced production of cytokines after polyclonal stimulation but found no significant effect of immunomodulation to both Th1 and Th2 responses to allergens or other stimuli. Even though no direct investigation on the effect on the vaccine response was determined, they concluded that probiotics may have immunomodulatory effects on vaccine responses
[30]. In a different study, in which infants received a cereal supplemented with
Lc. paracasei F19 from 4 to 13 months of life, there was an increased response to the diphtheria vaccine, with no effect on other types of vaccines administered to the infants
[31]. Soh et al. demonstrated a positive influence on the anti-HBsAg responses in infants supplemented for the first 6 months of life, again, with a mixture of probiotics,
Lc. rhamnosus LPR and
Bifidobacterium longum. However, this trend was not statistically significant, and no effect was observed in a modified vaccination schedule
[32].
Finally, an Australian group utilized the LGG strain only in the pre-natal period, administering supplementation to mothers from 36 weeks to delivery in comparison with a placebo. No variation in the diversity of the microbiota of the offspring
[33] and in the incidence of eczema
[34] was observed. Furthermore, in the same study group, Licciardi found that supplementation during pregnancy, starting from 36 weeks of gestational age with LGG, produced a significant reduction in the immunological response to tetanus, HiB and pneumococcus vaccines. The authors also reported an increase in regulatory T cells. This was a high-risk allergy cohort, and effectively, more infants in the group of mothers treated with LGG developed eczema or atopy
[35]. The authors speculate that the response to vaccines may have been influenced more by the atopic status of the offspring rather than by the effect of LGG supplementation. In fact, in the atopy status, the predominantly Th2-biased response could downregulate the Th1-based IgG levels that usually follow vaccination
[35].
3. Conclusions
In conclusion, the use of probiotics as adjuvant factors in vaccination is a subject of great interest but is still under debate. It could represent a strategic argument for the application of vaccines, both in the veterinary as well in the medical fields. The biological plausibility of the adjuvant effect, at least for some specific bacterial strains, is supported by clear demonstrations from both in vitro and in animal models. A recent review
[36] listed the potential mechanisms supporting the adjuvant action of probiotics, which modifies the microbiota composition. The authors suggested that microbe-associated molecular patterns (MAMP) and metabolites such as SCFA are the most probable candidates as promoters of the adjuvant action.
The huge differences observed between studies in the timing of intervention with probiotic supplementation (when and for how long), characteristics and age of the patients (pregnant women, newborn infants, adults, the elderly, healthy or high-risk subjects), the probiotic strain utilized and the type of vaccine (live attenuated, killed/inactivated and subunit/recombinant) greatly influence the conclusions.
We believe that further research studies are mandatory in this field, as the in vitro data so far available provide a solid rationale of use and yield some clues about the mechanisms of action, strongly suggesting that the use of probiotics as adjuvants should be considered in vaccination. Probiotics provide a relatively inexpensive mode of intervention to improve vaccine efficacy and the duration of protection
[22].
Specific attention has to be given to the strain used, since the efficacy of action is absolutely strain-related. Lactobacilli and LGG, in particular, are the most studied and promising among the probiotics widely utilized to date for these aims.
In this COVID-19 pandemic, nutritional supplements such as probiotics with antimicrobial and immunomodulatory activities are promising therapeutic adjuvants for the treatment of COVID-19 and for the prevention of viral spread
[37]. The present COVID-19 pandemic situation underlines how all efforts that can ameliorate vaccine efficacy should be attempted and can be a stimulus to promote further research. Since different vaccine platforms are used in COVID-19 vaccine development, we believe that the use of probiotics in this development should be considered. Indeed, probiotic supplementation as adjuvants in boosting immunity and in enhancing vaccine-specific responses could be important in the general population and, in particular, in the elderly and in children, where the effectiveness and duration of immunization could have even more bearing.