1000/1000
Hot
Most Recent

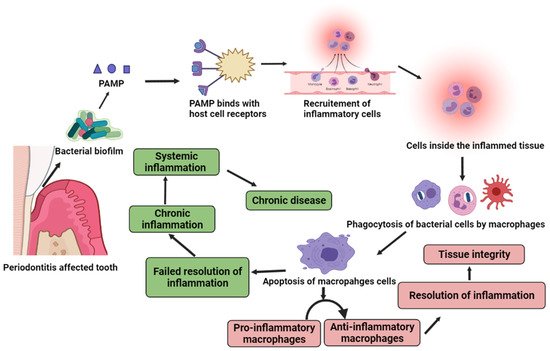
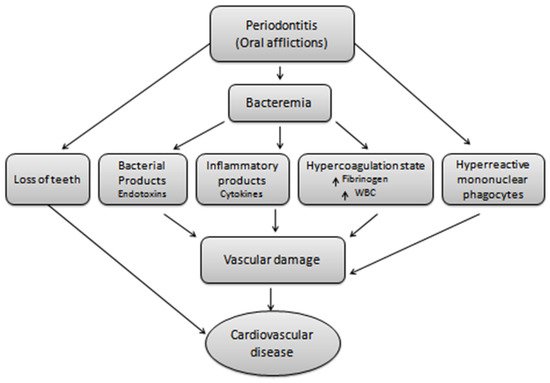
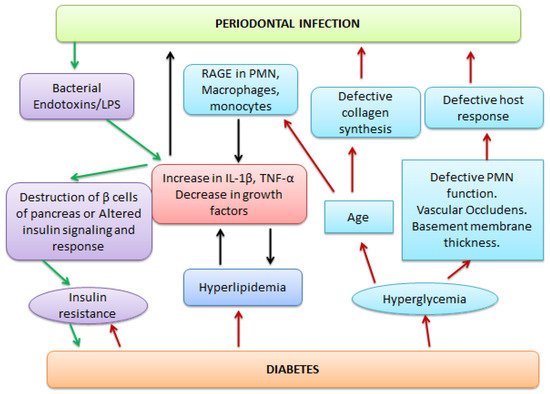
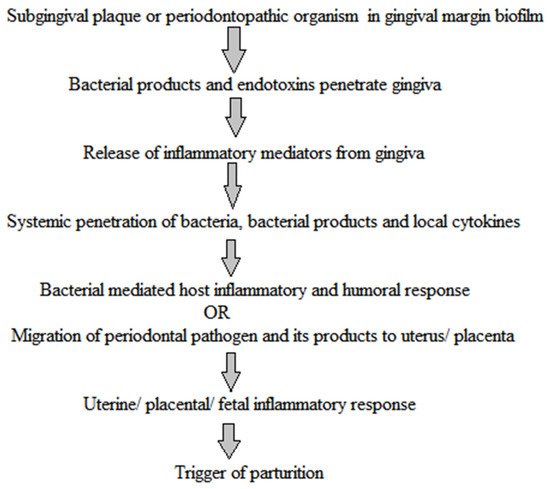
Periodontitis, a major oral disease, affects a vast majority of the population but has been often ignored without realizing its long-fetched effects on overall human health. A realization in recent years of its association with severe diseases such as carditis, low birth weight babies, and preeclampsia has instigated dedicated research in this area. In the arena of periodontal medicines, the studies of past decades suggest a link between human periodontal afflictions and certain systemic disorders such as cardiovascular diseases, diabetes mellitus, respiratory disorders, preterm birth, autoimmune disorders, and cancer. Although, the disease appears as a locoregional infection, the periodontal pathogens, in addition their metabolic products and systemic mediators, receive access to the bloodstream, thereby contributing to the development of systemic disorders. Mechanism-based insights into the disease pathogenesis and association are highly relevant and shall be useful in avoiding any systemic complications.
| Formulation | Components/ Devices |
Intervention/ Condition |
Description | Observations | Reference |
|---|---|---|---|---|---|
| Nanofibrous scaffolds | Silver nanoparticles, AgNPs and hydroxyapatite nanoparticles, and HANPs electrospun to prepare nanofibrous composites based on polylactic acid/cellulose acetate (PLA/CA) or polycaprolactone (PCL) polymers | Periodontal tissue and bone regeneration | Biodegradable electrospun nanoparticles-in-nanofibers-based scaffolds for guided tissue regeneration (GTR) and guided bone regeneration (GBR) with enhanced mechanical properties, cell adhesion, biocompatibility, and antibacterial properties | In-vitro studies of nanofibrous films cut into 10 × 10 mm2 samples showed that the addition of HANPs improved the cell viability by about 50 and AgNPs provided sustained antibacterial activity (40 mm zone of inhibition diameter) for 32 days. Additionally, the nanofibrous scaffolds offer optimum mechanical properties, tensile modulus (20–38 MPa), and a desirable degradation profile (40–70% of its mass in eight weeks). | [57] |
| Mineralized nanofiber | PLGA–collagen–gelatin coupled with calcium-binding bone morphogenetic protein 2 (BMP-2) |
Alveolar bone regeneration | Mineralized nanofiber segments (20 μm) coupled with BMP-2, mimicking peptides for periodontal bone regeneration | In animal studies, the mineralized nanofibers were implanted into critical-sized maxillary defects of 2 mm in diameter and 2 mm in depth in rats and a sustained release profile was recorded for over four weeks. X-ray microcomputed tomography (μ-CT) analysis revealed ~3 times greater new bone volume and mineral density. | [58] |
| Polymeric fibers | Electrospun nanofibers encapsulation BAR using poly (lactic-co-glycolic acid) (PLGA), poly (L-lactic acid) (PLLA), and polycaprolactone (PCL) either as a single or blended solution with polyethylene oxide (PEO) | Inhibition of Porphyromonas gingivalis and adherence to Streptococcus gordonii | Rapid-release polymeric electrospun nanofibers against P. gingivalis/S. gordonii biofilms in-vitro | The most promising formulation of 10:90 PLGA: PEO of electrospun nanofibers has demonstrated a 95% BAR release after 4 h, a dose-dependent inhibition of biofilm formation (IC50 = 1.3 μM), disruption of established dual-species biofilms (IC50 = 2 μM), and maintenance of high-cell viability. | [59] |
| Nanofibrous membrane | PLLA/gelatin | Periodontal tissue regeneration | Biodegradable multifunctional nanofibrous membrane prepared by electrospinning biodegradable polymers with magnesium oxide nanoparticles (nMgO) for periodontal tissue regeneration and high antibacterial capacity | In-vitro results showed that incorporating nMgO into poly (lactic acid) (PLA)/gelatin elevates the tensile strength to maintain structural stability and adjust the degradation rate for periodontal regeneration. Considerable antibacterial and osteogenic properties were also observed. The in-vivo investigations in a rat periodontal defect model demonstrated effective periodontal tissue regeneration guided via nMgO-incorporated membranes. | [60] |
| Polymeric films | PLLA/PCL blends containing propolis | Guided periodontal tissue regeneration | Biodegradable composite membranes produced from PCL/PLLA blends with a natural antibacterial extract (propolis) as novel periodontal barrier membrane | The in-vitro antibacterial studies revealed remarkable activities against Staphylococcus aureus (17 mm zone of inhibition). The prepared films also showed faster degradation in physiological conditions. | [61] |
| Biopolymer composite film | Curcumin | Topical patches for wound care, periodontitis, and oral cancer treatment | Multifunctional biopolymer composites based on curcumin-loaded bacterial cellulose/alginate/gelatin | The in-vitro studies have shown substantial antibacterial activity against E. coli and S. aureus infection. The purported composite films exhibited cytotoxicity to human keratinocytes and human gingival fibroblasts, and also show potent anticancer activity in oral cancer cells. | [62] |
| Regenerative scaffolds | Cellulose hydrogels and biopolymers derived from plants, and Larrea tridentate | Periodontal tissue regeneration | Cellulose hydrogel films enriched with LT for biomedical application in wound healing and as regenerative scaffolds | For in-vitro studies, NIH3T3 mouse embryonic cells were used for the measurements of cell viability and morphology assays. For in-vivo assays, hydrogel films were implanted intramuscularly into female Wistar rats (250 g weight; 2 months), to analyze their cytocompatibility and biocompatibility. | [63] |
| Nanofibrous membrane | PLGA/gelatin, dexamethasone (osteogenic), and doxycycline hyclate (anti-bacterial agent) | Guided bone regeneration | Bi-layered electrospun composite nano-membrane with combined osteogenic and antibacterial properties for guided bone regeneration | In-vitro studies indicated that both dexamethasone and doxycycline hyclate followed a favorable sustained drug release profile. The cell viability evaluation suggested good cytocompatibility. The osteogenesis analyses demonstrated an enhanced osteoinductive capacity for rat bone marrow stem cells, increased alkaline phosphatase activity, enhanced calcium deposition, and upregulated osteocalcin expression. Furthermore, the antimicrobial experiments revealed effective antibacterial potency. | [64][65] |
| Hydrogel | Polyacrylic acid (PAA) hydrogel containing metronidazole | Therapeutic dressing | Gamma-ray irradiation targeted metronidazole-loaded PAA hydrogel | The in-vitro cytocompatibility test was performed according to ISO 10993-5 and the formulation exhibited no cytotoxicity. The antibacterial activity against E. coli (ATCC 43895), S. aureus (ATCC 14458), and S. mutans (ATCC 25175) yielded satisfactory results. In release studies, metronidazole from the PAA hydrogel was consistently released and reached approximately 80% at 120 min. | [66] |
| Hydrogel | Doxycycline/ lipoxin and poly isocyano peptide (PIC) |
Periodontal | Antimicrobial and anti-inflammatory thermo-reversible hydrogel for improved gingival clinical attachment and periodontal drug delivery | The formulations were characterized in-vitro and in dogs with naturally occurring periodontitis. The results showed that the prepared hydrogel could be easily injected into periodontal pockets due to the thermo-reversible nature of the material. The formulation yielded significant release with no local or systemic adverse effects. A reduced subgingival bacterial load, pro-inflammatory interleukin-8 level, and improved gingival clinical attachment by 0.6 mm was also observed. | [67] |
| Liposomal gel | Lidocaine/ prilocaine |
Periodontal | A randomized, double-blind, cross-over, and placebo-controlled clinical trial of liposomal gel (intra pocket) for non-invasive anesthesia in scaling and root during periodontal therapy | The sample size calculation was based on pain intensity (primary outcome) using visual analogue scale (VAS) data. The study reported no difference between intervention groups concerning pain frequency/intensity (primary outcome). The anesthetic gel did not interfere with the hemodynamic parameters (secondary outcome). However, the above observations have a few limitations: First, there is no ideal scale for measuring pain and hence further clarification is necessary. Second, periodontal procedures usually cause low or moderate pain. Third, there was low patient compliance as many did not prefer local anesthesia. | [68] |
| Gel | Doxycycline encapsulated in β-cyclodextrin | Periodontitis | A randomized, blinded clinical trial to compare the effects of 10% doxycycline gel with doxycycline encapsulated in β-cyclodextrin gel on 33 subjects with periodontitis for 30 days. | The adjunctive topical agents (doxycycline encapsulated in β-cyclodextrin gel) along with scaling and root planning resulted in significant improvements in clinical periodontal parameters such as visible plaque index, measurement of periodontal probing depth, clinical attachment level, and bleeding on probing. | [69] |
| Hybrid hydrogels | Mesoporous silica, minocycline, silver, and gelatin methacrylate | Periodontal infection | Near-infrared light (NIR)-activated hybrid hydrogels | The hybrid hydrogels showed controllable minocycline delivery with increased release rates (in-vitro). The hydrogels also exhibited synergistic antibacterial activity (90%) against Porphyromonas gingivalis. The photothermal treatment was as high as 66.7% against P. gingivalis as well to rapidly eliminate and maintain low bacterial retention in the periodontal pockets. Furthermore, the in-vitro cytotoxicity studies revealed an 80% cell viability. | [70] |
| Hydrogel nanoparticles | Minocycline, zinc oxide, and serum albumin | Periodontitis | Broad-spectrum hydrogel-based minocycline and zinc oxide-loaded serum albumin nanoparticles for periodontitis application with low toxicity and high antimicrobial and antibacterial activity | In in-vitro analysis, a slow-release time was observed. Encapsulation efficiency was 99.99%. The in-vitro skin adhesion experiment showed a bioadhesive force of 0.35 N. Broad-spectrum antimicrobial and antibacterial ability and high cell survival rates with low toxicity were observed. | [71] |
| Microspheres | PLGA, PIC, doxycycline, and lipoxin | Periodontal infection | A tunable and injectable localized system based on PLGA microspheres, containing doxycycline and lipoxin, dispersed into thermo-reversible PIC hydrogel for personalized periodontal application | The in-vitro efficacy and bioactivity of the released doxycycline had presented a comparable zone of inhibition with respect to fresh or unbound drugs against gram-negative anaerobic bacteria Porphyromonas gingivalis (ATCC 33277). The fluorescent bead internalization assay of lipoxin revealed that more fluorescent beads were internalized that may stimulate RAW264.7 macrophage (Gibco) phagocytosis. The in-vivo test on ten 8-week-old male Wistar rats (~250 g) had exhibited no obvious inflammatory responses. |
[72] |
| Combination gel | Chlorhexidine and metronidazole | Gingivitis | A triple-blind randomized clinical trial on 90 subjects to compare and assess 0.8% metronidazole gel, 0.2% chlorhexidine gel, and the alternate application of the two gels against dental plaque and gingivitis for 14 days | The primary outcome measures are the bleeding index. The secondary outcome measures are the oral hygiene index, probing depth, and gingival index. The aforementioned outcomes were compared after 2 and 6 weeks. | [73] |
| Chitosan templates | Chitosan, glutaraldehyde, and doxycycline hyclate | Periodontal tissue regeneration | Cross-linking doxycycline-loaded freeze gelated chitosan templates for periodontitis | The in-vitro analysis of chitosan templates through a conventional dialysis sac method showed a 40 μg/mL of release after 24 h. Such a suitable drug release rate will also limit the toxicological effect of the cross-linking agent. | [74] |
| Retraction gels | Epinephrine, tetrahydrozoline, oxymetazoline, and phenylephrine | Gingival retraction | In-vitro vaso-constrictive retraction agents against primary human gingival fibroblasts in periodontal tissues | Immunocytochemical analysis revealed the biological effect of retraction gels on the expression of collagen types I and III. The generation of the reactive oxygen species triggered by the retraction gels indicated oxidative stress similar to the control cells using the dichlorofluorescein (DCF) fluorescent probe. | [75] |
| In-situ gel | Doxycycline hyclate, shellac, ethocel, and eudragit RS | Periodontitis | In-situ forming gels for localized periodontal pocket delivery. | The in-vitro release study through a dialysis membrane follows a sustained release pattern. It also exhibited in-vitro degradability and the antimicrobial effect against S. aureus, S mutans, E. coli, P. gingivalis, and C. albicans. | [76] |
| Nanofiber based hydrogel | Cellulose, κ-carrageenan oligosaccharide, surfactin, and herbmedotcin | Periodontitis | Anti-microbial loaded cellulose nanofiber and κ-carrageenan oligosaccharide composite hydrogels for strong antibacterial activity against periodontal pathogens such as Streptococcus mutans, Porphyromonas gingivalis, Fusobacterium nucleatum, and Pseudomonas aeruginosa in periodontitis treatment | Purportedly, they reduce the reactive oxygen species (ROS) generation, transcription factor, and cytokine production in human gingival fibroblast cells (HGF) under inflammatory conditions. | [77] |
| Hydrosilver gel | Silver | Chronic periodontitis | A prospective longitudinal pilot study using polymerase chain reaction analysis of hydrosilver gel against dental plaque in chronic periodontitis | The in-vivo model of chronic periodontitis was used for 15 days. The LAB®-test (LAB s.r.l.®, Ferrara, Italy) detected and quantified the presence and level of the most involved periodontitis pathogens that constitute the ‘red complex’: P. gingivalis, Tannerella forsythia and Treponema denticola. Other bacteria of the ‘orange complex’ were also monitored, such as Fusobacterium nucleatum, Campylobacter rectus, Aggregatibacter actinomycetemcomitans, Atopobium rimae, Eubacterium saphenum, Porphyromonas endodontalis, and Treponema lecithinolyticum, as the main components of microbiological shift. | [78] |
| Microporous annealed particle (MAP) hydrogels | Poly(ethylene) glycol | Tissue engineering and regeneration | Versatile new platform for the delivery of human periodontal ligament stem cells and periodontal tissue regeneration | In-vitro characterization revealed excellent retention, proliferation, and spreading of platelet-derived growth factors and human periodontal ligament stem cells within hydrogels. | [79] |
| Exosomal nanoparticles | Ginger phosphatidic acid | Oral biofilms | Plant-derived nanoparticles to inhibit P. gingivalis biofilm | Demonstrated inhibition of P. gingivalis induced bone loss and pathogenicity in an in-vivo mouse model of chronic periodontitis | [80] |
| Mesoporous nanospheres | Ipriflavone | Periodontal infection | Ipriflavone-loaded mesoporous nanospheres for periodontal augmentation | Periodontal augmentation was observed in an in-vitro osteogenesis model (MC3T3-E1 osteoprogenitor cells). | [81] |
| Nanocomposites | Chlorin e6 | Periodontal diseases | A photodynamic therapy-guided bioactive nanocomposite containing chlorin e6 as a photosensitizer against biofilms on dentin squares. The dentin samples were prepared from extracted caries-free human molars that serve as the substrates for biofilm formation. | Photosensitizer effect on Porphyromonas gingivalis, Prevotella intermedia, and Fusobacterium nucleatum and their corresponding biofilms on dentin squares 5 × 5 mm (thickness of about 1 mm). | [82] |
| Nanocomplexes | Bovine serum albumin | Periodontitis | Nanocomplexes for enhanced osteogenic differentiation of inflammatory periodontal ligament stem cells | The in-vitro hemolysis assay and in-vivo cytocompatibility assay using BALB/c mice (8 weeks old) revealed the high transfection efficiency and biocompatibility of the prepared nano complexes. | [83] |
| Carbon quantum dots | Tinidazole and metronidazole | Oral biofilms | Periodontitis treatment by penetrating the P. gingivalis biofilm and destroying its related genes | An in-vitro biofilm penetration assay revealed that nanoscale tinidazole carbon quantum dots can penetrate through the biofilm to induce significant inhibition of P. gingivalis under the biofilm. In addition, as exhibited in the in-vitro antibacterial assay, tinidazole carbon quantum dots impair toxicity and inhibit the major virulence factors and related genes involved in the biofilm formation of P. gingivalis. | [84] |
| Nanoplatelets | Fluoride | Periodontal bone tissue regeneration | Osteogenic differentiation of human dental follicle stem cells for tissue regeneration | MTS assay and cellular morphology analysis demonstrated low cytotoxicity of prepared nanoplatelets at low concentrations. | [85] |