1000/1000
Hot
Most Recent

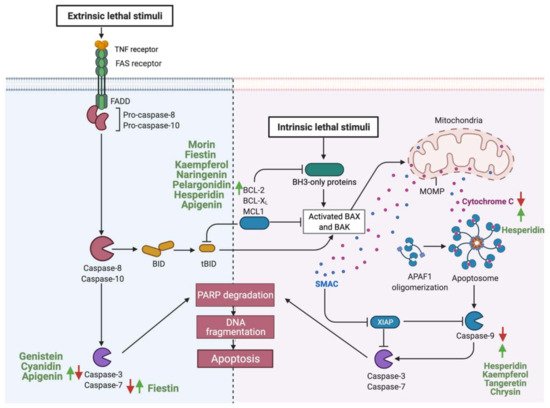
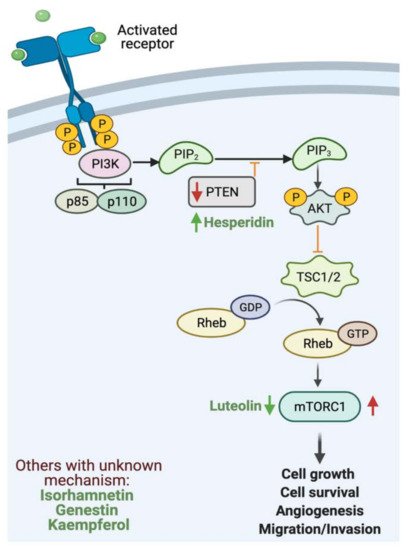
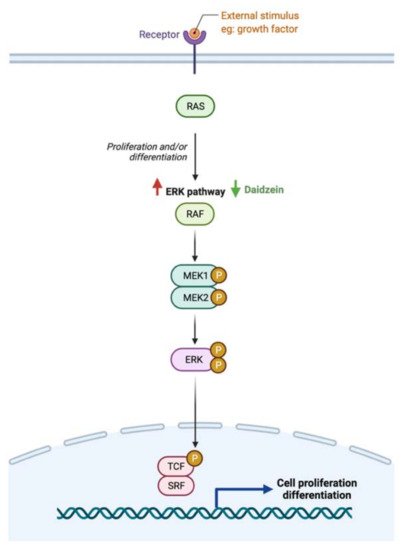
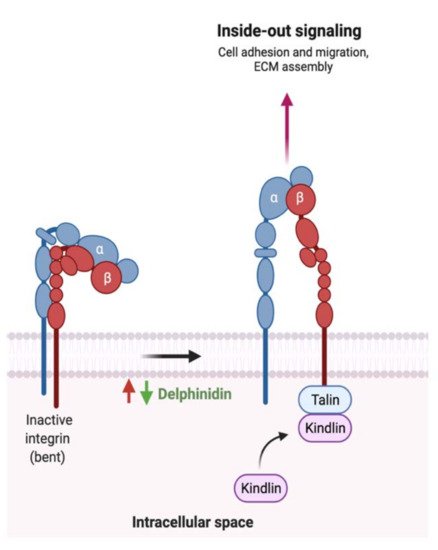
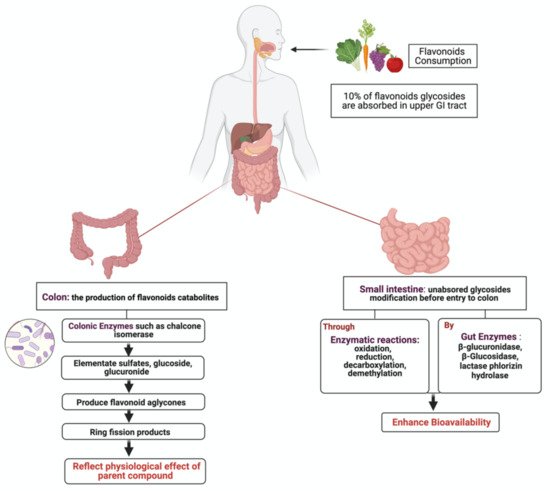
Gastrointestinal (GI) cancer is a prevalent global health disease with a massive burden on health care providers. Internal and external factors such as obesity, smoking, diet (red meat), low socioeconomic status and infection with Helicobacter pylori are the critical risk factors of GI cancers. Flavonoids are natural phenolic compounds found abundantly in fruits and vegetables. Upon ingestion, 90% of flavonoids consumed require further enzymatic metabolism by the gut microbiome to enhance their bioavailability and absorption. Several epidemiological studies reported that consumption of flavonoids and their enzymatic conversion by gut microbes is strongly associated with the reduced risk of GI cancer development. This review summarizes the current knowledge on the enzymatic conversion of flavonoids by the human gut microbiome. It also addresses the underlying anti-GI cancer effects on metabolic pathways such as apoptosis and cellular proliferation. Overall, metabolites produced from flavonoid’s enzymatic conversion illustrate anti-GI cancer effects, but the mechanisms of action need further clarification.
| Flavonoid Subclass | Name of Flavonoid | Structure of Flavonoid | Dietary Source | Metabolites Produced by Gut Microbiota | Bacteria Involved in Metabolism | Enzymes Involved in Metabolism | Site of Metabolism | Conversion Mechanism | Effect of Microbiota on Flavonoids | Model Used | References | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| In Vivo | In Vitro | ||||||||||||
| Flavonol | 1. Rutin | 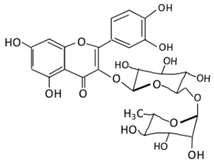 |
Lemons, berried, limes and oranges |
Quercetin -3- O- glucoside Quercetin |
Lachnoclostridium Eisenbergiella Escherichia Parabacteroides Erysipelatoclostridium |
α-rhamnosidases β-glucosidases |
Colon | Hydrolysis of rutin to remove sugar moiety | Permit the absorption of the aglycone Enhance bioavailability |
10 fecal samples from healthy individual following omnivore diet. |
[27][28][29][30][31] | ||
| 2. Fisetin | 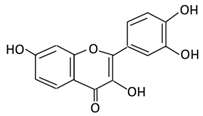 |
Persimmon and onions | No available data | [32][33] | |||||||||
| 3.Kaempferol | 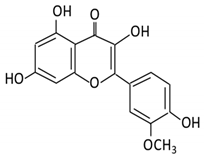 |
Tea, berries and cruciferous vegetables |
Kaempferol -3-O- glucoside p-coumaric acid kaempferol 3-(4 hydroxyphenyl) propionic acid 3-phenylpropionic acid |
Lactobacillus paracasei A221 |
β-glucosidases | Intestinal tract | (A)Degradation through multiple chemical reactions: - Deglycosylation - Reduction - Dehydroxylation (B) Hydrolysis |
Permit the absorption of the aglycone Enhance bioavailability |
Mice | 3 fecal samples from healthy individual Caco-2 intestinal barrier model |
[34][35][36][37] | ||
| 4. Quercetin | 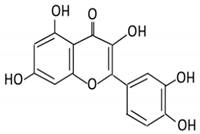 |
Black currants, cherries, apples and chokeberries |
4-hydroxybenzoic acid 3,4-dihydroxyphenylacetic acid 3,4-dihydroxybenzoic 3-(3-hydroxyphenyl) propionic acid |
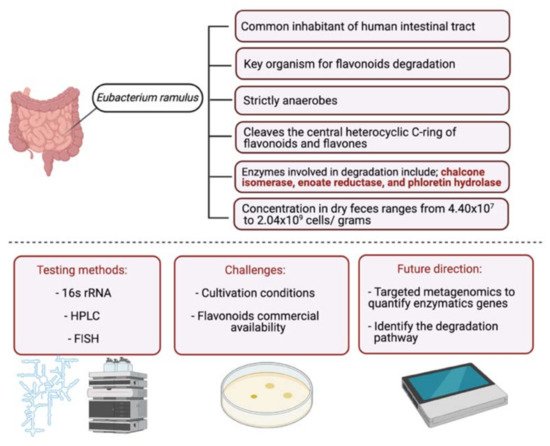
Bacteroides fragilis Clostridium perfringens Eubacterium ramulus Streptococcus S-2 Lactobacillus L-2 Bifidobacterium B-9 Bacteroides JY-6 |
Lactate phlorizin hydrolase | Small intestine |
Deglycosylation | Enhance bioavailability | Mice | 87 fecal samples from healthy elderly individual |
[38][39][40][41][42][43] | ||
| 5. Isorhamnetin | 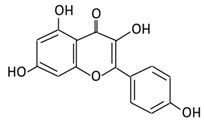 |
Ginkgo biloba and Hippophae rhamnoides |
3-O-neohesperidoside Isorhamnetin -3- glucoside Aglycone isorhamnetin |
Escherichia Enterococcus Bacillus. |
Not identified | Small intestine |
Deglycosylation | Permit the absorption of the aglycone | Rats | 1 fecal sample from healthy female |
[44][45] | ||
| 6. Morin | 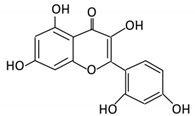 |
Psidium guajava, Prunus dulcis (Almond), chlorophora tinctoria and fruits |
Morin glucuronides Morin sulfates |
No available data | [12] | ||||||||
| Flavanones | 7. Hesperidin | 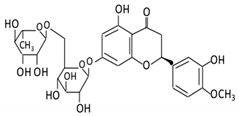 |
Orange citrus aurantium | Hesperetin | Not identified | Rutinose | Large intestine |
Cleaves the attached rutinose moiety |
Permit the absorption of the aglycone Enhance bioavailability |
fecal/ blood samples from 18 Lewis male rats |
[46][47][48][49] | ||
| 8. Naringenin | 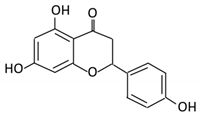 |
C.aurantium (chinese herbs) and grapefruit | Aglycone naringenin | Ruminococcus gauvreauii Bifidobacterium catenulatum Enterococcus caccae Eubacterium ramulus |
Chalcone isomerase | Large intestine |
Remove sugar group | Permit the absorption of the aglycone Enhance bioavailability |
fecal samples from healthy individuals |
[50][51][52] | |||
| 9. Eriodictyol | 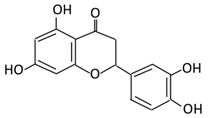 |
Lemon (Eriocitrin), Torr, Eridictyon californicum, | Eriodictyol 3,4-dihydroxyhydrocinnamic acid Phloroglucinol |
Parabacteroides distasonis Bacteroides uniformis JCM 5828 |
Chalcone isomerase | Colon | O-Deglycosylation | Permit the absorption of the aglycone Enhance bioavailability |
fecal samples from healthy individuals | [53][54][55] | |||
| Isoflavones | 10. Genistein | 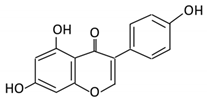 |
Fava and soy beans | Dihydrogenistein 6-hydroxy-O-desmethylangolensin 2-(4-hydroxyphenyl) propionic acid |
Lactobacillus Eubacterium ramulus |
Lactate phlorizin hydrolase | Small intestine |
Fermentation by anerobic bacteria | Permit the absorption of the aglycone Enhance bioavailability |
Samples from C57BL/6 female mouse |
[56][57][58][59] | ||
| 11. Daidzein | 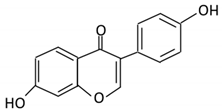 |
Soybeans, nuts and soymilk | Dihydrodaidzein O-desmethylangolensin S- equol |
Clostridium-like strain | β- glucosidase Lactate phlorizin hydrolase |
Colon | Reduction | Permit the absorption of the aglycone Enhance bioavailability |
Mice | [60][61][62] | |||
| Anthocyanins | 12. Cyanidin | 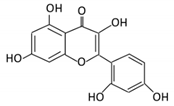 |
Bilberry, blueberry, grapes, blackberries, hawthorn | Cyanidin-3- glucoside | Clostridium saccharogumia Eubacterium ramulus |
Combined activity of bacteria and host enzymes | Small intestine |
Degrade polyphenolic glycosides |
Permit the absorption of the aglycone Enhance bioavailability |
Dawley rats | Fecal samples from healthy individuals | [63][64][65] | |
| 13. Delphinidin | 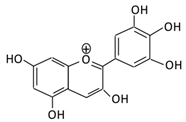 |
Dark grapes, eggplant, berries, red cabbage, | Gallic acids | Lactobacillus | β-d-glucuronidase β-d-glucosidase α-rhamnosidase α-galactosidase |
Colon | Cleavage of glycosidic bonds | Permit the absorption of the aglycone Enhance bioavailability |
Mice | [66][67][68][69][70] | |||
| 14. Pelargonidin | 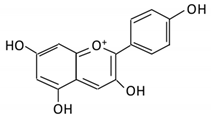 |
Bilberry and ficus bengalensis Linn | 4-hydroxybenzoic | Lactobacillus | β-d-glucosidase β-d-glucuronidase α-galactosidase α-rhamnosidase |
Colon | Cleavage of glycosidic bonds | Permit the absorption of the aglycone Enhance bioavailability |
Mice | [71][72] | |||
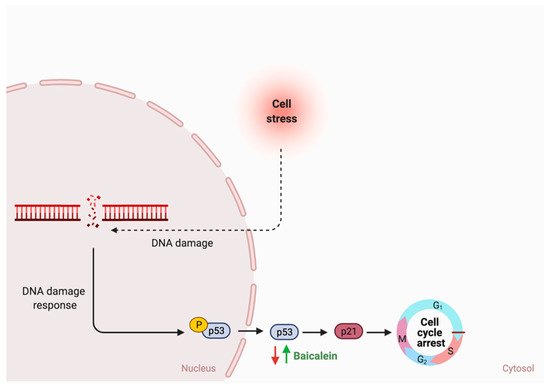
| Flavones | 15. Baicalein | 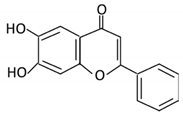 |
Scutellaria lateriflora L | Baicalein | E. coli | β-glucuronidase | Intestine | Hydrolysis to remove moiety |
Permit the absorption of the aglycone Enhance bioavailability |
Mice | HCT-166 cells SW-480 cells |
[73][74][75][76] | |
| 16. Luteolin | 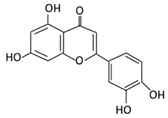 |
broccoli, celery and parsley, | No available data | [12] | |||||||||
| 17. Diosmin | 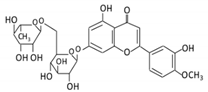 |
Citrus fruits | Diosmetin | Not identified | α- glucosidase β- glucosidase |
Small intestine |
Hydrolysis | Enhance bioavailability | Blood samples from healthy participants |
[77][78][79][80] | |||
| 18. Apigenin | 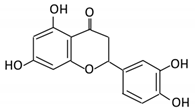 |
Tea, chamomile, parsley and oranges |
3-(4-hydroxyphenyl) propionic acid Apigenin |
Bacteroides distasonis Eubacterium ramulus Clostridium orbiscindens |
β- glucosidase lactase-phlorizin hydrolase |
Small intestine |
Glucuronidation Hydrolysis |
Enhance bioavailability |
Rats | Fecal and urine samples | [81][82][83][84] | ||
| 19. Tangeretin | 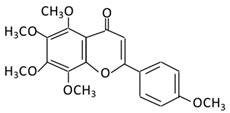 |
Poncirus trifoliate L, citrus fruit | Tangeretin-O-glucuronides | Lactobacillus Bifidobacterium |
Possibly by: A) rhamno glucosides B) C- glycosyl |
Small intestine |
Demethylation | Permit the absorption of the aglycone |
Rats | [85][86] | |||
| 20. Wogonin | 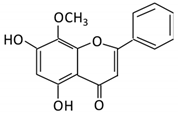 |
Scutellaria baicalensis Georgi | Wogonin | Not identified | β-glucuronidase | Intestine | Hydrolysis | Enhance absorption and bioavailability | Sprague-Dawley rats | [87][88][89][90] | |||
| 21. Chrysin | 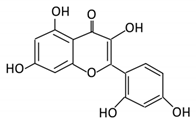 |
Honey | Chrysin glucuronides | Flavonifractor plautii ATCC 49531 | Flavone reductase |
Intestine | Reduction | catalyzes the hydrogenation of the C2–C3 double bond | ATCC 49,531 strain | [91][92][93] | |||