1000/1000
Hot
Most Recent

Synthetic dyes are mostly derived from petrochemical compounds, they are commercialized in liquid, powder, pastes, or granule forms. They are endowed with multiple potentialities such as fast and consistent coloration with different classes of fabrics as mentioned in the above section, a wide range of color pigments and shades, facility of manipulation, stability over several external factors and economical energy consumption. Therefore, the majority of synthetic dyes cause harmful impacts when discharged in non-treated or partially treated forms in the environment.
The worldwide developmental process influenced all fields of life by providing rapidity, efficacy and comfort. However, it has also engendered side effects related to biosphere pollution coming from uncontrolled pollutant discharge from all sorts of industries, especially those manipulating harmful and recalcitrant compounds [1]. Particularly, the dye industries generate huge amounts of hazardous wastewater routinely [2]. Dyes are used for the coloration of several materials such as textile fibers, paper, cosmetics, tannery, leather, food, pharmaceutical products, etc., [3][4]. Before 1856, dyes were derived from natural sources only. The increasing demand and excessive costs of natural dye extraction engendered the discovery of the first synthetic dye aniline (mauveine) in 1856 by a chemist named Perkin [5]. This purple dye gave a stable and uniformly distributed color when applied to silk [6]. Dying industries depended on synthetic dyes ever since and started to expand globally, attaining nearly 8 × 105 tons of synthetic dyes produced per year [7][8]. Notably, the textile industry accounts for ~75% of the global dyestuff market and involves around ten thousand different dyes used for printing and/or coloring multiple types of fabrics [9][10]. Textile industries are mostly located in developing countries such as India, Bangladesh, Sri Lanka and Vietnam, where they enhanced employment capacity, building the economy and foreign exchange earnings [11][12]. However, these countries do not fully respect effluent discharge norms because of their poor wastewater treatment systems. They often reject large quantities of untreated or partially treated dye effluents, eventually resulting in huge environmental pollution [13][14]. Thus, our study aims to give a comprehensive survey on textile synthetic dyes and the discharged effluents of textile industries. It focuses on introducing synthetic dyes and their classification in the first part. It delimits the impacts of these dyes on the ecosystem and human beings in the second part and discusses the available treatment methods of the released textile industry wastewater in the last part.
They use dyes in different ways on textile fabric (Figure 1). Bleaching is the removal of dye color (decolorization) from textile fibers, and finishing comprises crosslinking, softening and waterproofing [7]. Natural dyes, which have been known ever since ancient times, are derived mainly from plants; and synthetic dyes are artificially synthesized from chemical compounds. These are cellulose fiber dyes, protein fiber dyes and synthetic fibers dyes [4][15].
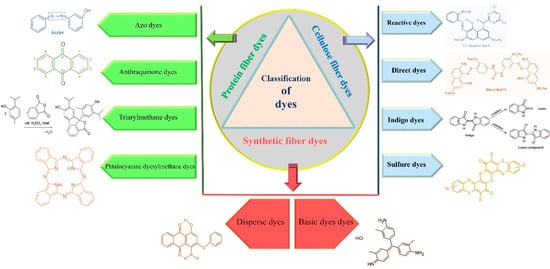
Cellulose fiber originates from plants such as linen, cotton, ramie, rayon, lyocell and hemp (Figure 1). These types of fabrics give perfect dyeing results with reactive dyes, direct dyes, indigo dyes and sulfur dyes [16].
Reactive dyes constitute the major class of cellulose fiber dyes and work well with some protein fibers (Figure 1). They are known for their high pigmentation, permanent effect, facility of manipulation under a wide temperature range and versatility due to diverse reactive groups able to form covalent bonds with multiple fibers [10][14].
Direct dyes are very affordable, yet tend to remain in an aqueous form rather than binding to cellulose fibers (they can be used with certain synthetic fibers as well). Thereby, they are combined with inorganic electrolytes and anionic salts in the form of sodium sulfate (Na2SO4) or sodium chloride (NaCl) to enhance their fabric binding capacities (Figure 1). Thus, it is recommended to wash them in a cold cycle and with fabrics of the same color [17].
The indigo or dark blue color belongs to the classification of vat dyes, which are originally not soluble in water but became soluble after an alkaline reduction (Figure 1). The textile dyeing process occurs with the water-soluble or leuco form of indigo, then this form oxidizes under air exposure and returns to its original insoluble or keto form to ensure a perfect bonding of the dye to the fabric. The indigo dyes are mostly used in blue denim dyeing, which explains their production in huge amounts around the world [18][19].
Sulfur dyes constitute a small, yet important class due to their excellent dyeing properties, ease of application and low cost (Figure 1). They have a complex structure with a disulfide (S–S) bridge. They belong to the vat dye classification; thus, they are reduced from the keto to the leuco form via sodium sulfide utilization. Leuco sulfur becomes soluble in water to achieve the dyeing purpose [20][21].
Protein fibers such as silk, cashmere, angora, mohair and wool originate from animal sources (Figure 1). They are susceptible to high pH levels; hence they are dyed using a water-soluble acid dyestuff to obtain a molecule of an insoluble dye on the fiber [22]. Acid dyes encompass azo dyes as the most important group followed by anthraquinone, triarylmethane and phtalocyanine dyes [23][24].
Azo dyes account for the largest category (60–70%) of the total synthetic dyes industry due to their versatility, cost-effectiveness, simplicity of utilization, high stability and high intensity of the color [25][26]. This dye has a prominent chromophore (-N = N-) structure, ensuring the solubility of the dyes in water and its attachment to the fiber [27][28]. Azo dyes are classified into three groups (mono, di and poly) depending on the number of azo groups in their structure (Figure 1). These groups are attached to an aromatic or heterocyclic compound on one side and an unsaturated heterocycle, carboxyl, sulphonyl, or aliphatic group on the other side [29][30].
The class of anthraquinone is extensively used in textile dyeing industries; the red dyestuff particularly has been used for a long time [31]. These dyes are known for their solubility in water, bright colors and excellent fastness properties (Figure 1). The antharaquinone structure could constitute junctions with azo dyes [32][33].
The triphenylmethane dyes are widely applied in the textile industry for either dyeing wool and silk protein fibers when formed of two groups of sulfonic acid (SO3H). They can be used as indicators if they contain only one sulfonic acid (SO3H) auxochrome in their chemical structure (Figure 1). These dyestuffs are known for their solubility in water and their wide and intense color range [16][34].
The phtalocyanine family of dyes is synthesized by a reaction between the 1,4-Dicyanobenzene compound with a metallic atom (Nickel, Cobalt, Copper, etc.) to produce green and blue shades (Figure 1). They have multiple inherent properties such as good colorfastness to light, resistance to oxidation, solubility in water and chemical stability [35][36].
Synthesized fibers are composed of spandex, polyester, acrylic, polyamide, polyoacetate, polypropylene, ingeo and acetate fabrics (Figure 1). They are used in 60% of global fiber production due to their wide application range. These fibers are dyed using direct dyes, basic dyes and disperse dyes [37][38].
Disperse dyes are the smallest molecules among all dyes. These dyes are insoluble in water but stable under high-temperature exposure (Figure 1). The high-temperature dyeing solution is a mixture between the dyestuff powder and the dispersing agent [39][40][41].
Basic dyes are also called cationic dyes because they transform into colorful cationic salts responsible for dyeing the anionic fiber textile [42]. These dyes are susceptible to light; thus, they are strictly used for dyeing paper nylon and modified polyesters. Their principal structures are cyanine, triarylmethane, anthraquinone, diarylmethane, diazahemicyanine, oxazine, hemicyanine, thiazine and hemicyanine [10] (Figure 1).
Synthetic dyes are mostly derived from petrochemical compounds, they are commercialized in liquid, powder, pastes, or granule forms [15]. Therefore, the majority of synthetic dyes cause harmful impacts when discharged in non-treated or partially treated forms in the environment [43][44]. Hence, a huge volume of improper discharge is rejected continuously [45][46]. Dye effluents contain high biological and chemical oxygen demand (BOD and COD) and they are very rich in organic and inorganic pollutants such as chlorinated compounds, heavy metals, sulfur, nitrates, naphtol, soaps, chromium compounds, formaldehyde, benzidine, sequestering agents and dyes and pigments [47][48].
Liquid and solid wastes discharged from textile industries contain dyes, plastic, polyester, fibers, yarns and other hazardous materials as mentioned in the above section (Figure 2). These polymeric compounds have been responsible for the pollution of local landfill habitats and agricultural fields, especially in developing countries. This soil pollution engenders plant growth inhibition by causing oxidative stress, lowering the protein content, photosynthesis and CO2assimilating rates [7][49].
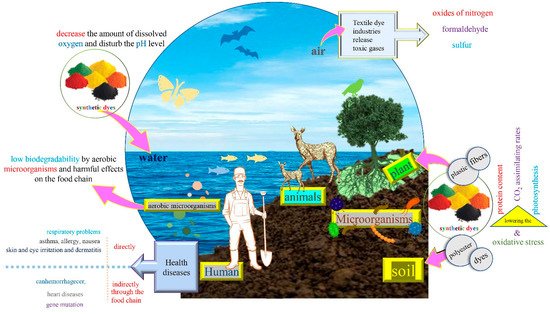
Textile dye industries release toxic gases like sulfur, formaldehyde, oxides of nitrogen, volatile compounds, particulate matter and dusts distinguished by an unpleasant smell (Figure 2). This air pollution could affect humans (workers and customers), animals, the final product and the environment [50][51][52].
The wastewater contains multiple toxic materials and its color result from the discharge of several dyes; it is noticeable and very recalcitrant even in low concentrations ( , yet the average concentration of textile effluent dye reaches about 300 mg/L [53]. These factors lead to several ecological impacts on the aquatic system such as the inhibition of photosynthesis in aquatic plants, low biodegradability by aerobic microorganisms and harmful effects on the food chain [54][55]. Water is highly susceptible to pollution compared to the other areas, and it is also hard to determine the pollution level in aquatic systems.
Dye products and by-products existing in wastewater discharge or the dust produced inside the textile industry pose serious damages and long-lasting health impacts to human beings (Figure 2). They affect several vital organs (brain, kidney, liver, heart) and systems (respiratory, immune, reproductive) of the human organism [56][57]. Diseases may occur either directly through inhalation such as respiratory problems, asthma, allergy, nausea, or skin and eye irritation and dermatitis, or indirectly through the food chain such as tuberculosis, cancer, hemorrhage, gene mutations, and heart disease [2][58].
Textile industries use huge amounts of water in the dyeing processes, making it hard to treat the enormous quantities of wastewater discharge (Figure 3). Therefore, several countries have imposed rules to reach a standard before the effluent is released into the ecosystem or is reused for other purposes. For this to happen, physical, chemical and biological technologies have been employed to protect the environment and human health from discharge problems. These strategies could be implemented solely or in combination to obtain effective results [13][29][59].
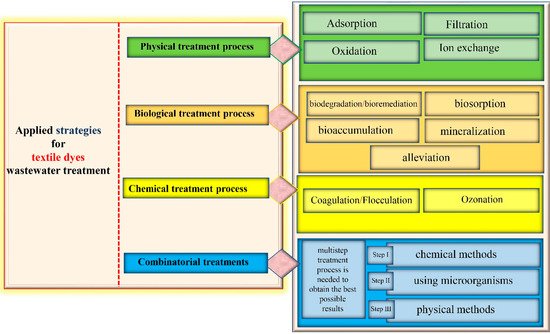
The physical treatment (Figure 3) of industrial wastewater involves conventional processes such as adsorption, filtration, ion exchange and oxidation [15].
The achievement of dyes decolorization by adsorption with activated carbon was extensively studied. For instance, malachite green was adsorbed with curcuma-based activated carbon [60], with tetraethylenepentamine activated carbon [61] and with carbon coated layered double hydroxide [62]. Rhodamine B was successfully adsorbed by ordered mesoporous carbon and commercial activated carbon and by treated rice-husk based activated carbon 4 adsorption occurred via the activated carbon.
Nanotechnology is the science of nanoscale materials (size ≤ 100 nm); it has attracted numerous researchers due to the great potentials of nanoparticles in removing textile dyes [63]. The shape, size, structure, purity and arrangement of nanomaterials matters in the process of decolorization [64]. Nanoparticles are mainly applied directly to wastewater to adsorb rejected dyes on their surface for further elimination [65]. Multiple scientific works covered dye degradation using nanocomposites including graphene oxide/zinc oxide (GO/ZnO)
Filtration is a membrane-based separation process (Figure 3) that involves popular techniques such as reverse osmosis, ultrafiltration and nanofiltration, to allow the acquisition of reusable water and recycled dyes [66][67][68]. The concept of these procedures consists of transporting industrial wastewater throughout several membranes differing by mesh size and separation mechanisms to finally obtain clean water [69][70]. Dye waste water treatment by the membrane filtration method was reported by [71][72][73][74][75][76][77][78][79][80][81][82][83][84][85][86][87][88][89][90][91][92][93][94], he used nano-membrane filtration. [95] coupled electro-fenton reaction with membrane filtration to achieve better results.
Ion-exchange adsorbents are introduced in the wastewater in either solid or liquid form, they are used to bind harmful anions or cations of the opposite charge, and in turn, release an equivalent amount of non-harmful hydrogen ions [96][97]. The review paper of Hassan and Carr [98] addressed strategies for reactive dye removal by applying the ion-exchange method [98][99][100]. Raghu and Basha (2007) combined the ion-exchange with chemical or electrochemical methods to remove dyes from textile wastewater [101]. This technique is great for the elimination of toxic and soluble pollutants (Figure 3) from effluent water; however, its use has been limited because of its high cost [102].
Advanced oxidation processes (AOPs) have been extensively applied for textile dye degradation due to their powerful ability to oxidize a wide range of synthetic dyes and other complex pollutants existing in textile effluents [103][104]. They include catalytic oxidation, which is the process of active radical production (hydroxyl or sulfate radicals) on a particular catalyst surface [105][106]. This reaction occurs in an acid medium (pH ~ 3) to allow the decomposition of the hydrogen peroxide into hydroxyl free radicals acting as strong oxidants [107]. The main drawbacks of the oxidation technique are the generation of hazardous by-products in cases of incomplete oxidation and the possibility of sludge formation [108][109].
The most common chemical treatment processes (Figure 3) are coagulation, flocculation and ozonation [53]; they are utilized for contaminant elimination and in particular, those released in textile wastewater [110].
Coagulation and flocculation (Figure 3) are the simplest chemical methods for the pretreatment of textile dye effluent [111][112]. This technique enables the elimination of suspended insoluble materials by adding charged chemical colloids (Aluminium sulfate (Al2(SO4)3), Iron (III) chloride (FeCl3), Iron (II) sulfate (FeSO4), alum, lime, etc.) provoking coagulation and settling with oppositely charged particles in the polluted water [113]. Acid red 73 was treated by coagulation [114] and
This approach uses ozone as a strong oxidizer, its cleaning properties allow it to eliminate toxic textile effluent compounds such as azo dyes [115][116]. Other benefits of ozone, when used in the gaseous state (Figure 3), are no fluctuation of the volume of wastewater and no generation of sludge [117]. In this respect, the literature has multiple examples on the degradation of dyes including Reactive red 120 [106] The main shortcomings of this process are that it is highly susceptible to many factors (pH, salts, temperature, etc.), it can release toxic compounds and its cost is elevated [113].
This process could also be induced on a laboratory scale by isolating and screening appropriate microorganisms, succeeded by a scale up to allow for textile effluent treatment and decolorization [3][118][119]. It is noteworthy to cite the role of extracellular laccase produced by multiple fungi [120] also mentioned the ability of few filamentous fungi in the treatment of crystal violet and methylene blue dyes [71][72][73][74][75][76][77][78][79][80][81][82][83][84][85][86][87][88][89][90][91][92][93][94][95][121][98][99][100][122][123][101][124][125][126][127][128][129][130][131][132][133][134][135][136][137][138][139][140][141][142][143][144][120][145][146][147][148][149][150][151]. proved to be useful in azo dyes degradation [120][152].Lysinibacillussp.
All the above-mentioned treatments proved effective in alleviating textile wastewater toxicity (Figure 3). It combines several treatment methods, starting with chemical methods to allow for the elimination of solid contaminants [16][153][154]. The secondary treatment is achieved using microorganisms to reduce the COD and BOD rates, to remove turbidity and to convert the generated sludge from the primary treatment into non-harmful products [155]. In the tertiary treatment, physical methods are applied to ensure the total decontamination of the textile wastewater and its safe reuse or release in the environment [156].