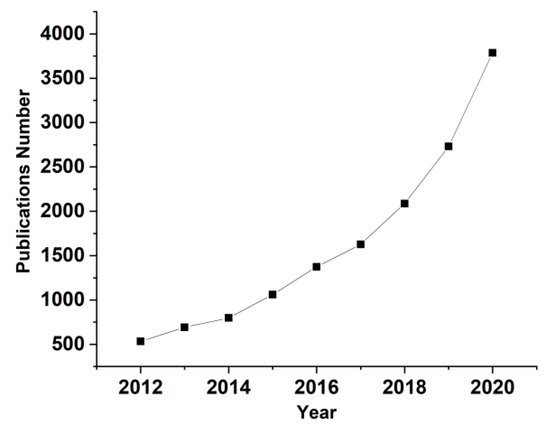
PVA is a hydrophilic polymer with excellent film forming properties as well as good processability and mechanical strength for film/membrane fabrication, but the major disadvantage of PVA based film for opto-electronic applications is its higher water absorption. To prevent such a problem, a possible solution is coating PVA film with protective layers to eliminate water absorption from the environment when no direct air/polymer interface is required; for example, triacetyl cellulose (TAC) film is used as a protective PVA based polarization layer in liquid crystal display (LCD)
[43]. Other studies have reduced intrinsic polymer hydrophilicity. Using thermal crosslinking and adding different additives, an optimistic conclusion has been reached, namely that PVA has great potential for use in the synthesis of composites/films, as underlined in
[44]. shows the effects of PVA film treatments on the optical and mechanical properties of the final compound.
PVA thermally degrades at temperatures just exceeding 200 °C, at which temperature it often sees the onset of melting and crystallizes from the melt so rapidly that 100% amorphous samples cannot be obtained. In
[59] it is shown from the heat capacity increment curve vs. mass fraction crystallinity (well fitted by the two-phase model comprising crystalline and mobile amorphous phases) that there is no evidence for the existence of a third phase, namely a rigid amorphous phase. In semicrystalline polymers (with two phases) crystalline and amorphous regions have no sharp boundary, allowing one macromolecule to run across the two regions. We can treat the PVA polymer as a crystalline lattice with voids filled by amorphous matter
[60].
In general, PVA films with no special treatments have a degree of crystallization of approx. 30–40%
[48], which can be increased gradually only by annealing the films, to 60% (at a maximum of 150 °C, where, most likely, one sees the chemical destruction of the polymer, the hydroxyls are lost, and polyene and other chemical structures are formed
[45]). When adding different dopants, like 0.1 mol/L lithium chloride, the crystallization is reduced to 18%
[45], or with chitosan (to 15%), and with supplementary glycerol down to 1%
[48].
A highly crystallized molecular structure of final PVA films generates a polymer with low mechanical strength and water resistance performance
[46]. Pure PVA is semi-crystalline, and the degree of crystallinity can be controlled by the film/gel method, by polymer blend with a plasticizer (resins, rubber and elastomers)
[48][61][62], or even by dopant insertion. For example, the lower crystallinity of the PVA films with the addition of various conductive ions may be due to the strong interaction among the polymer molecules and conductive ions, as with Ni
2+ [47], or ferric ions Fe
3+, when the crystallinity of PVA is reduced from 41.6 to 7.7% with the addition of 2.0% ions
[46].
The presence of acetate groups affects the ability of PVA to crystallize upon heat treatment
[63], which is of particular interest for crosslinked hydrogels. The crystalline degree of PVA can be improved by repeated cycles of freezing and thawing, and by gamma ray irradiation
[56]. An alternative method to control crystallinity is the use of derivate polymers, such as acrylamide (a carcinogenic compound) modified PVA, prepared by the alcoholysis of vinyl acetate and acrylamide copolymers. This enhances the water solubility and tunes the tacticity (with effects on the physical properties of the polymer) and crystallinity
[64].
It is known that aqueous solutions of PVA gradually undergo gelation upon standing at room temperature (from the formation of networks in which the PVA crystallites generated by spinodal decomposition serve as the junction points)
[65]. For gel formation through freezing/thawing methods, as the number of cycles is increased, the number and stability of crystallites also increases (due to the condensation of the PVA solution by the formation of ice)
[66]. Low-temperature crystallization is the most popular method used to prepare PVA gel, with excellent mechanical properties, but it uses toxic dimethylsulfoxide (DMSO) as a solvent
[67]. A simpler and more eco-friendly method for preparing transparent PVA hydrogel with an enhanced crystallinity, with water as the only solvent, uses swollen PVA placed into a hot-pressing machine (95 °C), pressed from 2 to 20 MPa (in successive steps from 2 to 15 min), and kept at room temperature without drying for one week (for gelation) followed by drying in air for two days, followed by vacuum drying for 2 days. The gel thus prepared shows a decrease in net crystal size and good mechanical properties
[68].
Other methods to improve the specific properties of PVA for different applications involve blending it with other polymers: with poly(vinylpyrrolidone) PVP
[49][50][69] for increased thermal, electrical, mechanical stability and electrochemical properties; with polyethylene glycol (PEG) for facilitated gas transport
[70], with PVAm in order to improve film formation and mechanical properties; reinforcing it with carbon nanotubes
[52] or with poly(glucosyloxyethyl methacrylate) (poly(GEMA)) for the suppression of the thermal decomposition of PVA in an aqueous solution, and to increase the thermal decomposition temperature above 300 °C
[51].