1. Introduction
Carbon plays a unique role in electrochemical catalysis and energy storage systems or sensors. Its contribution to conductivity and its intrinsic capacitance has been explored in capacitors and batteries, alone and in combination with other components
[1]. In particular, the additional faradaic contributions from other components in nanostructured hybrid materials have been crucial to develop new nanostructures and also to improve supercapacitor electrodes as nicely reviewed in
[2][3][4]. Furthermore, when in form of graphene or nanotubes (CNT), nanocarbons show added electrochemical features with respect to graphitic carbons
[5].
From a biological point of view, carbons have been shown to be good substrates for cell growth
[6][7], and in the case of nanocarbons like carbon nanotubes or single-layer graphene coatings, also allow sensing, drug delivery, imaging and local electrostimulation
[8][9]. However, despite all expectations derived from graphene high conductivity, no evidence of charge capacity in isolated nanocarbons has been given. Electrostimulation has neither been possible to date in those cases, despite the reported interactions. Carbon nanotubes on the other hand have mixed reports of biocompatibility. It is worth noting that CNT are known to enter cells, evidencing phagocytosis, which allows labelling in medical applications, and are toxic in some reports
[10]. On the other hand, any type of nanoparticle ends up within cells, with a variety of responses. While this is useful for detection of cancer cells, the time viability of healthy cells is not yet known.
In some cases, simplified interpretations of the role of nanocarbons have been given, like the possible explanation that graphene surfaces modify the mobility of K
+ ions and in turn enhance neuron excitability
[11] While possible, no proof exists of that, since potassium is present mostly internally in cells and the extra cellular space has higher concentrations of other ions. In fact, previously, it has been shown that it is Ca
2+ concentration the main factor modifying the direction of neural growth
[12].
Furthermore, the conducting character of carbon and nanocarbon particles is significant beyond the usual reports. Induced electrical dipoles in conducting materials are basically ignored in most works related with neural development. However, recent works have shown
[13][14][15] that conducting materials immersed in cell cultures undergo dipole formation in presence of external fields. Such dipoles, in turn, induce also additional effects in cell behavior and cell growth, and open the possibility of remote electrostimulation protocols. Despite the similar physical wireless effects, each type of material has a different effect on neurons, even with similar conductivity and redox behavior. Thus, PEDOT-PSS (Poly(3,4-ethilendioxithiophene)-poly(estyrene sulfonate)) conducting polymers modify neurite growth direction, while IrO
x enhances the speed of dendrite growth.
[13]. Although the possible wireless effect is present for any conducting material, refs
[13][14][15] it is not yet known why different materials differ in the type of effects on neural cells.
On the other hand, what experiments suggest
[16] is that the properties of nanoparticles, including carbon-graphene, may be different in bulk as part of nanostructured materials than in isolated form. Nanoparticles in suspension migrate to the interior of cells, and for that reason are interesting labelling agents and therapeutics for cancer, but such migration would be hindered if nanoparticles are part of a hybrid material. Therefore, beyond the intrinsic effects for nanocarbons, or the effect related with their size, conforming them into nanostructured materials offers a new route to interact with biological systems. To start, bulk nanostructured materials may allow the formation of micro and macro electrodes with specific exposed surface areas, and porosity that allows cell oxygenation and vascularization.
Surface exchange effects may be modified by enhancing surface area, and so three-dimensional scaffolds have been created based on carbon-based structures such as graphene oxide or reduced graphene oxide
[17]. These 3D structures allow growth and vascularization through them, and may reach eventually a good electrical connection that allows stimulation. Carbon fibers have also been used in solid arrays with conducting polymers coating them, although the final electrochemistry seems to be only that of the polymer. Although conducting polymers may seem an alternative, either as support of carbon or as coating, neither the biocompatibility nor the electrochemical behavior is improved by forming the composite
[18].
Electrostimulation experiments have not been reported yet in either of those nanocarbon cases as far as we know, neither on 3D carbon scaffolds or carbon fibers, although sensing has been possible with single nanotubes
[7]. Usual electrostimulation has been carried out clinically with bare metals (steel, platinum, and TiN)
[19], coated in some cases with Iridium oxide with a fast electrical response not found in carbons.
Electrodeposited IrO
x is, on the other hand the best substrate for neural growth
[19][20] and with good conductivity. When used as coating for those basic electrodes an enhancement of charge capacity and decreasing inflammation in the biological tissue is observed. Recent studies have shown that IrO
x substrates favor the optimal adhesion of neurons and dendrite growth. It is suspected that redox intercalation of ions in the presence of electric fields, and ionic compositional gradients modify cell behavior
[21].
Conducting polymers have also been studied, with polypyrrole-X and PEDOT-X conducting polymers using various counterions, X,
[22], being X a biocompatible counterion. The idea is based on the fact that the conductivity of such polymers may offer an alternative to the conductivity of carbon materials, while allowing easy conformation as fibers or 3D substrates. However, the work has not offered yet the expected results. If X is the usual commercial PSS (polysterene sulfonate), contradictory results are found, depending on the adhesion layer used for cell adhesion (polylysine, collagen, etc.)
[18]. Additionally, different cell types behave differently on them. While primary mammalian neurons do not grow on PEDOT-PSS, astrocytes do
[18], and also xenopus neurons
[13]. Biocompatibility is clear for mammalian neurons; however, if aminoacids such as lysine are used as X counterions
[22], the role of X in the base polymer is evidenced.
Once materials with optimal compatibility are obtained, a crucial need for all those electrodes is a significant value of charge capacity, that allows a safe charge delivery while maintaining optimal biocompatibility in absence and in presence of electric fields. That may be achieved by the creation of a large surface area through formation of 3D structures as mentioned above that will offer an enhanced charge transfer at the surface, or through generation of nanostructures with several biocompatible components, that, in combination, show better properties than the sum from each component. In both approaches, an enhanced surface interacting with the biosystems and larger charge capacity are expected to decrease inflammation and electric field secondary effects.
As mentioned, graphene oxide and reduced graphene oxide have been prepared in macroscopic solid 3D forms using directional freeze drying processes, and evidencing significant compatibility effects and reinforcing neural cell growth and metabolism. However, in electrochemical terms, no significant conductivity or charge capacity has been achieved yet. On the other hand, the intrinsic properties of the starting graphene materials used are a key factor, and pristine graphene has properties above graphene oxides. In particular, pristine graphene has been prepared in a rather elegant way by electrochemical exfoliation of graphite both in absence and in presence of surfactants to stabilize the suspension
[23][24]. It is remarkable that in some specific media, using oxalic acid electrolytes, no surfactants are needed and suspensions of graphene are stable for years
[24]. To this date, that significant suspended graphene has resulted in smaller amounts than traditional GO Hummers preparation
[25] but may render interesting 3D forms in the future.
In addition to microstructure and charge capacities, materials used as electrodes have additional restrictions to retain biocompatibility during electric field application. It is crucial that the material hinders secondary radical formation reactions for example. Metals like Pt or stainless steel may be used as electrodes, but the electron-ion transfer at the surface induces H2O oxidation and O2 reduction, yielding to radical formation that results in enhanced inflammation and cell death. In vivo experiments show that the alive system protects itself from those effects by inflammation, encapsulating the implanted material, and eventually the implanted electrode needs to be removed.
An attractive alternative arises from the use of electroactive materials as electrodes, that allow redox intercalation within their structure, in a similar way to M
+ ion batteries, since such redox processes offer an alternative to radical formation in aqueous electrolytes. That is indeed the mechanism working in IrO
x (really an oxohydroxide that allows intercalation of H
+, Na
+ and K
+), polypyrrole or PEDOT polymers (allowing intercalation of cations and anions). Both material types have been studied as substrates for neural growth and as electrodes in electrostimulation
[19][26]. Their charge capacity has been enhanced by specific preparation processes of dynamic electrodeposition
[21][22] yielding one order of magnitude enhancement in charge capacity with respect to standard electrodeposited materials. However, it is possible in some cases to go beyond in charge capacity by nanostructuring the best materials in specific forms.
2. IrOx Basic Material: IrOx Basic Electrodeposition Process
As mentioned, IrO
x anodically deposited is among the best conducting substrates for neural growth. IrO
x, understood as a complex oxohydroxide of general formula K
1.7IrO
0.8 (OH)
2.2 1.8 H
2O, had been anodically deposited before
[20][21], and had distinguished from crystalline IrO
2 or from anodic oxidation of metallic Ir metal. Anodic deposition of precursor solutions based on slowly hydrolyzed IrCl
3 or IrCl
4 solutions in alkaline conditions yields IrO
x, yield coatings with poor adhesion and macroscopic cracks if constant current protocols are used
[21]. However, dynamic pulsed anodic deposition renders thin layers (170 nm for 50 cycles and 300 nm for 100 cycles) of IrO
x that are well adhered and have a one order of magnitude larger charge capacity
[21] than conventional IrO
x obtained by constant current deposition methods. The same dynamic deposition successful for IrO
x offers later additional mechanisms for the formation of hybrids. The final solid IrO
x coating is amorphous but contains K
+ in a reproducible stoichiometry. Chemical exchange of K
+ is possible and the ion is easily removed by soaking in water, and easily replaced by H
+ or Na
+.
It is worth remarking here that the nature of the Iridium precursor solution has been confusing in the literature
[27], and the formation of hybrids discussed below benefits from such discussion. The existence of a nanoparticle suspension has been claimed upon aging of iridium solutions, based only on data from high-voltage TEM equipment. However, electron diffraction data from iridium solutions and coatings also show the existence of redox processes occurring under the electron beam. The existence of UV-VIS charge-transfer redox exchange conferring the blue color to the hydrolyzed solutions involve mixed-valence polynuclear Ir oxo species resulting from a hydrolysis process, and not necessarily nanoparticles of IrO
2 [21]. Precipitation with excess K+ ions generates a solid with 2.2 K/Ir ratio, while anodic deposition yields a K/Ir ratio of 1.7 according to XPS
[21]. That means that deposition is not a flocculation or electrophoretic process, but a true redox electrochemical process. Electrochemical quartz microbalance study also shows additional features for the deposition
[21], where K
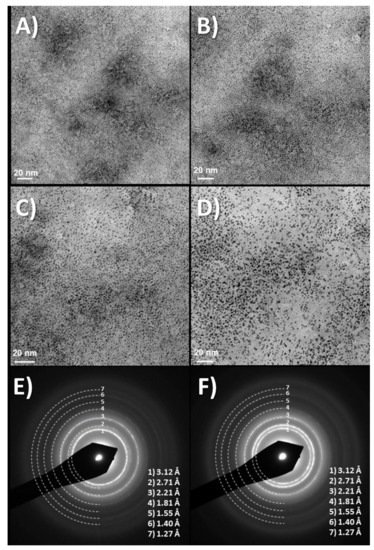
+ is intercalated and deintercalated depending on the voltage applied during the process. Dynamic light scattering (DLS) measurements of the solution, yield similar cluster sizes found in initial low-intensity TEM measurements (10–20 nm) instead of the 2 nm size found in high-voltage TEM, which is truly a redox modification to metallic iridium (see
Figure 1 and
Figure 2). Careful interpretation of the global data allows us to identify that the electron beam acts as an electrochemical cell reducing Ir-oxo species containing K
+ and OH
− to K
xIrO
2 and finally to Ir metal suspensions of 2 nm size nanoparticles, by a slow process for low-intensity electron beams, and very fast for high-intensity beams. Thus, the existence of iridium anionic oxoclusters in solution, formed during hydrolysis in a similar way to known polyoxometalates
[28], is a more coherent explanation than thinking about generic nanoparticles. Therefore, as suggested above, the deposition of IrO
x is not an electrophoretic pure process of suspended IrO
x particles but, as other electrodeposition processes, is a full redox oxidation process of iridium oxoclusters in solution yielding an amorphous oxohydroxide.
Figure 1. (
A–
D) Time evolution of TEM images at about 10 min intervals, showing TEM images of dry drops of Iridium oxo solutions obtained from hydrolysis of IrCl
3, evolve under the electron microscope (120 KV Jeol). Last two images, (
E,
F) Diffraction rings obtained at this low resolution match those of quasiamorphous K
xIrO
2 and later metallic Ir. Thermal evolution has also been observed before in Ar atmosphere (in O
2 yielding IrO
2 rutile
[21]) Images show two different time intervals. Global time in the order of minutes. (Original results).
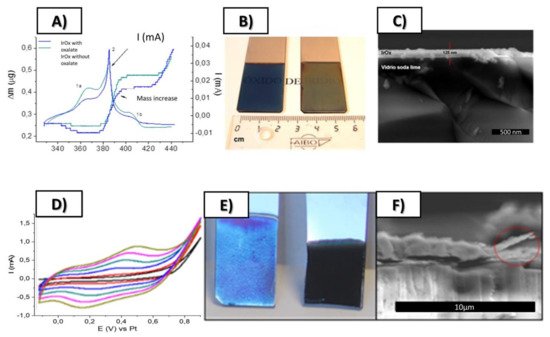
Figure 2. Top: (
A) Simultaneous cyclic voltammetry and ECQM mass changes during dynamic Electrodeposition of IrO
x involving mass deposition and K
+ intercalation/deintercalation, (
B) SEM lateral images of resulting coatings on Pt (12 nm) glass slides, (
C) Macroscopic images of IrO
x deposited on Pt (12 nm)-Ti (5 nm)-glass substrates. Bottom (
D) Typical electrodeposition Cyclic voltammetry of IrO
x-Nanocarbon hybrids (this case IrO
x-NGO) (
E) macroscopic images showing that the first layers are mostly IrO
x and (
F) SEM lateral images of the coatings. From ref.
[24][29] Published with permission of Elsevier.
That discussion is relevant in terms of possible nanocarbon IrOx hybrid formations, since the nanostructure is dependent on the interactions among both components. It is also relevant because, although carbons do not deposit in absence of iridium, the deposition of IrOx drives the deposition of carbons, by the existing interaction among them.