Mitochondrial dysfunction is known to contribute to mitochondrial diseases, as well as to a variety of aging-based pathologies. Mitochondria have their own genomes (mitochondrial DNA (mtDNA)) and the abnormalities, such as point mutations, deletions, and copy number variations, are involved in mitochondrial dysfunction. In recent years, several epidemiological studies and animal experiments have supported the Developmental Origin of Health and Disease (DOHaD) theory, which states that the environment during fetal life influences the predisposition to disease and the risk of morbidity in adulthood. Mitochondria play a central role in energy production, as well as in various cellular functions, such as apoptosis, lipid metabolism, and calcium metabolism.
1. Overview
Mitochondrial dysfunction is known to contribute to mitochondrial diseases, as well as to a variety of aging-based pathologies. Mitochondria have their own genomes (mitochondrial DNA (mtDNA)) and the abnormalities, such as point mutations, deletions, and copy number variations, are involved in mitochondrial dysfunction. In recent years, several epidemiological studies and animal experiments have supported the Developmental Origin of Health and Disease (DOHaD) theory, which states that the environment during fetal life influences the predisposition to disease and the risk of morbidity in adulthood. Mitochondria play a central role in energy production, as well as in various cellular functions, such as apoptosis, lipid metabolism, and calcium metabolism. In terms of the DOHaD theory, mtDNA copy number may be a mediator of health and disease. This paper summarizes the results of recent epidemiological studies on the relationship between environmental factors and mtDNA copy number during pregnancy from the perspective of DOHaD theory. The results of these studies suggest a hypothesis that mtDNA copy number may reflect environmental influences during fetal life and possibly serve as a surrogate marker of health risks in adulthood.
2. Mitochondrial Diseases
Since the recognition of mitochondrial diseases in humans in 1962
[1], numerous studies have elucidated the physiological and pathological significance of mitochondrial function. Mitochondria are intracellular organelles, which are present in almost every cell in the body and are responsible for the production of energy in the form of adenosine triphosphate (ATP)
[2][3]. Thus, in patients with mitochondrial diseases, organs that are dependent on mitochondrial production for energy may be damaged
[4]. In the neonatal period, a “triad” of cerebromuscular (e.g., recurrent apnea, inactivity, convulsions, and hypotonia), gastrointestinal and hepatic (e.g., vomiting, poor feeding, diarrhea, and hepatomegaly), and myocardial symptoms (e.g., arrhythmias, cardiomyopathy, and heart failure) related to mitochondrial diseases have been reported
[5]. In infancy, symptoms of mitochondrial diseases include poor weight gain, progressive liver damage, cerebellar ataxia, pyramidal tract signs, psychomotor disturbances, respiratory failure, myotonia, and amino aciduria
[6][7]. Central nervous system symptoms and muscular symptoms of mitochondrial diseases occur during childhood and adulthood. Three major mitochondrial encephalomyopathies have been reported: mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke-like episodes
[8][9][10]; myoclonic epilepsy and ragged-red fiber disease
[11][12]; and chronic progressive external ophthalmoplegia, including Kearns–Sayre syndrome
[13][14][15]. As the pathogenesis of mitochondrial disease suggests, the pathological significance of mitochondrial dysfunction is enormous. Mitochondrial dysfunction is also a hallmark of biological aging, as mitochondrial dysfunction worsens with age
[16].
In eukaryotic cells, nuclear DNA (nDNA) and mitochondrial DNA (mtDNA) cooperate to regulate cellular functions
[17]. Human mtDNA comprises a cyclic multicopy genome containing 16,569 base pairs, with usually tens to thousands of copies per cell. Human mtDNA is maternally inherited, as paternal mitochondria in fertilized eggs are removed by “mitophagy”
[18][19]. Mitophagy, a quality control mechanism, is the selective degradation of mitochondria by autophagy for maintaining the health of the mitochondrial network
[20][21]. Human mtDNA is independently replicated, and each genome mutates on its own, resulting in the formation of a condition called “heteroplasmy”
[22][23]. Heteroplasmy means the existence of a normal version of the maternal mtDNA genome and a version of the genome containing a mutation, and this mixing rate easily changes as cells proliferate and differentiate. The mode of existence of human mtDNA also differs among tissues
[24]. For example, mtDNA exists in single circles in many organs and cells, but in the heart, several mtDNA molecules are linked together in a complex network. The fact that the mode of existence of mtDNA differs in each tissue may be a consequence of the search for the most suitable state to maintain mtDNA in each tissue. While the mitochondrial genome is considerably smaller than that of the nuclear genome, the mRNA transcribed from mtDNA accounts for about 30% of total cellular mRNA in the heart and 5–25% of total cellular mRNA in other organs
[25]. After being transcribed, mitochondrial RNA is processed, the mitoribosome is assembled, and respiratory chain proteins encoded by mtDNA are synthesized
[26]. Human mtDNA at the population level is shaped by selective forces within the female germ line under nuclear genetic control
[27]. According to the literature, the incidence of mitochondrial diseases is 9.6 per 100,000, of which 1.2 to 1.5 patients have a single mtDNA deletion
[28][29][30]. The majority of mitochondrial diseases are inherited through autosomal or X-linked inheritance rather than maternal inheritance, and around 75% of mitochondrial diseases, especially those that occur in childhood, are caused by nDNA mutations
[6][31]. Around 300 causative genes of human mitochondrial diseases have been identified using next-generation sequencing
[32]. In an animal study, investigators removed the nucleus from a fertilized egg of a mouse with an mtDNA mutation and transplanted it into an unfertilized egg of a healthy female mouse
[33]. In this study, the resulting litter was healthy. However, as mentioned above, such a technical approach would only be applicable to a subset of patients with mitochondrial diseases. In terms of the drug delivery system (DDS), which delivers target molecules to mitochondria, various attempts have been made to protect or activate mitochondrial functions
[34]. Mitochondrial transplantation treatment
[34][35] is one of these treatment challenges, but several issues remain to be addressed
[35][36][37].
As shown in previous studies, the number of mtDNA copies in the peripheral blood decreases with age
[38], and the decrease is negatively associated with age-related events, such as all-cause mortality
[39]. In addition, mtDNA copy number loss is associated with cognitive function
[40], cardiovascular diseases
[41], and infectious morbidity and mortality in patients with chronic kidney disease
[42]. Mitochondrial DNA depletion syndrome (MTDPS) refers to diseases in which the mtDNA copy number is reduced, and mitochondrial energy metabolism is impaired
[43]. The underlying pathogenesis of MTDPS refers to abnormalities in genes related to the replication maintenance mechanism of mtDNA encoded in nDNA, resulting not only in mtDNA depletion but also in multiple deletions and point mutations
[44]. These include abnormalities in
POLG,
POLG2,
Twinkle,
RNASEH1,
DNA2, and
MGME1 genes
[45]. The mode of inheritance of MTDPS is autosomal recessive
[46]. As suggested by MTDPS associated with a variety of pathological conditions, dysregulation of mtDNA copy number and mitochondrial dysfunction play a role in a variety of pathologies. The maintenance of mtDNA copy number via mtDNA replication is of high importance not only at the cellular level but also at the individual level.
In terms of its physiological significance, mtDNA copy number is important from fetal, through infantile and childhood stages, to adulthood. In fact, mtDNA copy number influences early developmental cell differentiation and reprogramming of induced pluripotent stem cells
[47]. In a previous study, the mtDNA contents of newborns with abnormal birth weights were lower than those of newborns with normal weights
[48]. Based on accumulating evidence
[49][50][51], reduced mtDNA content may be a putative link between abnormal fetal growth and metabolic and cardiovascular complications in later life. In a recent population-based follow-up study of middle-aged Swedish women (
N = 2387), the mtDNA copy number was significantly lower both in women with type 2 diabetes and in those who developed type 2 diabetes during the follow-up period compared to those who did not develop type 2 diabetes
[52]. Furthermore, a study based on data from the U.K. Biobank confirmed that a higher mtDNA copy number was significantly associated with a lower prevalence of neurodegenerative diseases (odds ratio (OR) = 0.89, confidence interval (CI) = 0.83; 0.96) and a lower incidence rate of neurodegenerative diseases (hazard ratio = 0.95, 95% CI = 0.91; 0.98)
[53].
The Developmental Origin of Health and Disease (DOHaD)” theory posits that environmental factors during development act as risk factors for health and disease in adulthood
[54][55][56][57][58][59][60]. Considering the physiological and pathological significance of mtDNA copy number from the perspective of DOHaD theory could shed light on the potential role of mtDNA copy number as a biomarker of disease in later life
[61]. This paper discusses the potential of mtDNA copy number in early life as a biomarker of health and disease in later life (
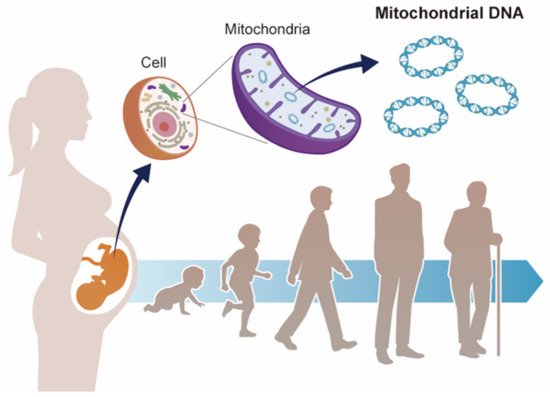
Figure 1), focusing on recent epidemiological studies that suggest a correlation between environmental factors and mtDNA copy number changes during the fetal period.
Figure 1. Mitochondrial DNA and developmental origins of health and disease (DOHaD). Mitochondrial DNA copy number increases or decreases in response to environmental changes. Environmental stress during fetal life may contribute to the risk of disease in adulthood via mitochondrial DNA copy number.
3. DOHaD Theory
The sum of chemical, physical, and biological stresses from the environment, namely the exposome, contributes to human health conditions
[62]. Studies by Barker on infants with low birth weights in the 1980s
[63][64][65] led to the development of the Fetal Origins of Adult Disease theory in 1990
[54]. Taking into account the results of subsequent epidemiological studies
[66][67], the DOHaD theory was proposed in 2004
[55].
Today, a number of epidemiological studies and animal model studies suggest that environmental factors in embryonic, fetal, neonatal, and early childhood periods are associated with health and disease in adulthood and old age, including the risk of developing chronic diseases, supporting the DOHaD theory
[68][69][70][71][72][73][74][75][76][77]. For example, in cases of food deprivation during pregnancy, the fetus acquires an energy-saving constitution in anticipation of the environment after birth, in which nutrient intake may be insufficient
[78]. If the mother has a glucose-intolerant pregnancy (gestational diabetes mellitus (GDM)) or is obese, it can also cause obesity in the child. It is thought that the fetus receives an excess supply of glucose from the mother, which causes insulin to be overproduced. In addition, the growth-promoting effects of insulin and insulin-like growth factors (IGFs) may be the cause of obese children (gigantism). In addition to nutritional status, various environmental factors, such as exposure to environmental chemicals (e.g., endocrine-disrupting chemicals), are risk factors for disease development
[79][80][81]. It is well known that passive smoke exposure during pregnancy interferes with fetal brain development
[82]. Low birth weight, especially intrauterine growth retardation, is known to be a risk factor for chronic kidney disease and hypertension. The kidneys are made up of nephrons (renal units), and there are usually around 2 million nephrons per kidney. However, low maternal nutrition, infections, and drugs (ethanol, gentamicin, NSAIDs) can cause the number of nephrons in the fetus to be extremely low, which may lead to the development of hypertension and chronic kidney disease after birth
[83]. Other factors such as delayed growth outside the uterus and postnatal administration of steroids and antimicrobials have also been suggested to be associated with the risk of decreased renal function, leading to renal disease. According to research, circadian rhythms during pregnancy also play a role in the health and well-being of children in adulthood, including their risk of particular diseases
[84][85]. In addition, gestational hypertension and gestational diabetes may lead to worse health outcomes in newborns
[86][87]. Previous studies have provided support for the roles of both the epigenome and gut microbiota during the early postnatal period in future health, thereby supporting the DOHaD theory
[88][89][90][91].
Based on the DOHaD theory, there is believed to be a link between exposure to external stress in the fetal environment and risk of noncommunicable diseases (NCDs) in adulthood
[92]. Given the increase in the proportion of the aging population in many developed countries, reducing health expenditure through the prevention of NCDs has become an important social and political issue. Previous research suggested that research focusing on the DOHaD was important to develop regional strategies to contribute to a reduction in the incidence of NCDs in low-income countries
[93].