1000/1000
Hot
Most Recent

Cell and gene therapies have been developing dramatically over the past decade. To face and adapt to the development of these new therapies, the Food and Drug Administration (FDA) wrote and updated new guidelines from 2016 and keep updating them. Mesenchymal stem cells (MSCs) are the most used cells for treatment, far ahead from the induced pluripotent stem cells (iPSCs), based on registered clinical trials at clinicaltrials.gov. They are widely used because of their differentiation capacity and their anti-inflammatory properties, but some controversies still require clear answers. Additional studies are needed to determine the dosage, the number, and the route of injections (location and transplantation method), and if allogenic MSCs are safe compared to autologous MSC injection, including their long-term effect. In this review, we summarized the research our company is conducting with the adipose stromal cells in engineering cell sheets and their potential application
Different types of stem cells are used for research and for translational medicine: embryonic stem cells (ESCs) [1], mesenchymal stem cells (MSCs) [2], and induced pluripotent stem cells (iPSCs) [3]. Ethical debates about the use of ESCs make their use more difficult for human application [4], even if clinical trials have been conducted recently with ESCs [4]. The iPSCs can be engineered by the transfection of four different factors into somatic cells [3], but as for the ESCs, the iPSCs have also raised ethical issues [5][6][7], and clinical trials are conducted all over the world, but mainly in the USA, China, Japan, and France [8]. The European Medical Agency approved cell therapies in the past few years: Chondrocelect was the first approved cell therapy in 2009 [9]. This review is not focused on presenting the different types of stem cells used for cell and gene therapies, but it is focused on the MSCs and cell sheet engineering with MSCs.
MSCs are very popular cells used in the research and over sixty-eight thousand publications involving the use of MSCs were published on PubMed, as of January 2021 [10]. MSCs are pluripotent stem cells that were discovered around 30 years ago [2], and they can be isolated from bone marrow, adipose tissue, Wharton’s jelly, periosteum, villous chorion, fetus, and dental pulp [2][11][12][13][14], and there are no ethical issues. In the organism, the function of the MSC is to support the structure of the organs but also to generate cells of the specific organ when it is required. They adhere quickly to the cell culture surface, and their morphology is fibroblastic. They can be cultured easily, and their stemness is characterized by the capacity of the MSC to self-renew and maintain the stemness properties, being passaged many times without karyotype alteration [15]. However, there is always a risk that MSCs could transform into sarcoma [16], requiring a long-term follow-up on preclinical animal studies and clinical trials, up to 15 years based on the Food and Drug Administration guidelines (FDA). MSCs curative properties and advantages can be divided in three different parts:
(a) They can differentiate into different types of cells (before or after transplantation), and the self-renewal property of the MSC is very important, but it is a critical characteristic that must be understood. Even with a self-renewal capacity, the aging of the MSC could be a major problem with an increase of mutation and loss of differentiation capacity [17][18]. In 2006, Dominici et al. published a list of the minimal criteria defining the MSC: MSC must express CD73, CD90, and CD105, and must lack the expression of CD14, CD19, CD34, CD45, and HLA-DR; in addition, MSCs must differentiate into cells originated from the three embryonic stem cell germ layers (endoderm, ectoderm, and mesoderm [19]) such as adipocytes, chondrocytes, and osteoblasts, as the most used [20].
(b) MSCs have anti-inflammatory potential and immune-modulatory properties, and promote cell growth and tissue repair, through the secretion of cytokines and extracellular vesicles [21]. In addition to this, the absence of HLA class II protein is a key factor, because MSCs could be used for allogeneic graft on patients, facilitating the use of MSCs in cell therapies. The activation of the HLA Class II leads to a rejection of transplanted cells or organs [22][23][24][25]. Functional MSCs do not express or express a very low level of HLA-DR (major histocompatibility complex class II, MHC II), meaning that MSCs have a lower immunogenicity than that of other cells [26][27][28][29]. In vitro studies showed that human bone marrow stem cells (BMSCs) are not recognized by T-lymphocytes but can suppress the proliferation of the T-lymphocyte [29]. For the past 20 years, human MSCs were used in animal studies, with successes, on the basis of the low probabilities that the xenotrans-plantation of human cells in animals will trigger an inflammatory response and the human MSC rejection. Human MSCs (hMSCs) were injected in different animals without any adverse events reported: mouse [30], rat [31], rabbit [32], zebrafish [33], swine [34], and dog [35], and as review for xenotransplantation of hMSCs [36]. This positive characteristic can be used for gene and cell therapy preclinical tests on animals before translational application, by using the MSCs that are planned to be utilized in the clinical trials (e.g., culture media, approved cells for clinical used by Federal Agencies). The absence or low immunogenicity of MSCs will allow their mass production, a better characterization, and the decrease of cost. In the other hand, MSCs act also as immunomodulators, by reducing inflammatory activity [37][38][39] and were used as a racehorse cure with no immunoreaction [40], for the bone repair of rats [41], in a human trial for Crohn’s disease [42][43][44][45][46], and for perianal fistula [47][48], as outlined in a review publication [49].
(c) An additional positive criterion is the large-scale manufacturing of the MSCs, which will provide enough cells for cell therapies [50, 51]. Typing the key words “bioprocessing, mesenchymal stem cells” in PubMed, there are only 160 publications referring to the large-scale production methodology of the mesenchymal stem cells. The bioprocessing of any stem cells must be well planned and controlled, including the determination of the donors (inclusion/exclusion criteria), the methodology of isolation, the type of culture media, and the processes for the mass production [50]. Positive and negative outcomes of stem cell therapy for animal studies and clinical trials can be related with the modification in the stem cells’ bioprocessing [51]. It is encouraged to work with MSC providers (or any other cells) that have an approved chemistry, manufacturing, and control for clinical trials, to facilitate the transition from preclinical to clinical trials; but it is also important to determine the cell culture conditions in the preclinical phase that will be used in the clinical trials to ensure that the data obtained in the preclinical studies and the methodologies will be approved by the federal agencies.
MSCs are the most used stem cells in clinical trials, and MSCs have shown promising hope for patients in need of gene and cell therapies [52], and over 1220 clinical trials have been conducted over the world (keyword: mesenchymal stem cell at clinicaltri-als.gov). Most of the clinical trials reached phase I and II, and very few of them reached phase III. MSCs can be easily isolated from different tissues. Even if bone marrow stem cells are still the MSCs used most often, the invasive procedure to isolate the bone marrow makes it more difficult and more stressful for the donors [53], compared with the use of adipose stem cells that can be easily isolated from liposuction [54][55]. In addition, the number of isolated adipose stromal cells from the liposuction can be 50,000 times higher than the number of bone marrow stem cells isolated from the bone marrow [56][57][58]. For all these reasons listed above, we decided to study the potential of the adipose stromal cells, a specific mesenchymal stem cell.
Engineering of tissues and organs with mesenchymal stem cells involves not only the stem cells but it could also involve biocompatible scaffolds, important for cell signaling stimulation and for transplantation [59][60]. The simplest and most economical methodology to treat patients with MSCs is the injection of isolated MSCs. On the other hand, a more complex approach consists in growing the cells in a 3D structure, using different methodologies for support, such as scaffolds and 3D printing. The efficiency of the cell therapy is based not only on the quality and the stem cell phenotype, but it is also related with the transplantation methodology of the cells.
Our company decided to develop cell sheets using mesenchymal stem cells, to target the cells on the damaged area, in absence of a specific scaffold, for different reasons: cheaper methodology, no additional step is necessary to prepare the culture dish to engineer cell sheet, absence of scaffold will not lead to fibrosis in the empty space left during the scaffold degradation and harvesting of cell sheet requires strong cell–cell connection and extracellular matrix. The use of cell sheet increases the cell survival and limits the potential ectopic biodistribution of the cells after transplantation, when the life span of injected single cells is very short (hours to weeks) [61][62][63]. The increase of the cell lifespan increases and extend the curative properties of the transplanted cells.
Biodistribution after cells injection/transplantation is a major concern for the federal agencies, especially to determine if the cells could be a threat to a patient’s health on a long-term treatment. When medications are absorbed by the patients, the medications are distributed, metabolized, and excreted after a certain period of time [64]. On the contrary, stem cell treatments are expected to or could have a very long-term effect. The timeline for the pharmacokinetics (PK) of cell treatment is different from that of the drugs PK, and cells should be followed up for an extended period, estimated to a year’s level. For a long term follow up, with our actual technology, only a genetic modification of the cells can be performed. At the best of our knowledge, there is no approved genetic cell modification to study over a long period of time the fate of the transplanted cells. Side effect of genetically modified cells will be a major concern that will require a large group of patients to determine the safety of the modifications. However, this approach has two advantages: it will be possible to have a long-term follow-up of the cells if the trackers are expressed by the cells and the cell viability could be confirmed.
The stability and the storage of the cell sheets is also another important part of the product development. As for the organs that must be discarded after a short period of time, those organ-based stem cells must be discarded if they are not used rapidly, but this problem could be overcome if the cell-based organs could be vitrified and stored for a long term in nitrogen liquid. As for many biological living products based on cells, the shelf life of the products can be short, especially if the cells are still in proliferation. It is then necessary to deliver the final product to the patients as soon as possible, but it is not always possible. The objective of the cell sheet vitrification is to build a bank of the cell sheets and transplant them, after the thawing test. Because of the low immunogenicity of the MSCs, this methodology will allow to have a large bank of cell sheets, available in a very short time for the patients, for allogeneic transplantation. Ohkawara et al. vitrified cardio-cell sheets and stored them in nitrogen liquid from 2 days to 3 months, without affecting the cell sheet morphology on a macroscopic level, and their cell therapy function, after transplantation on the heart. This not only means that the cell sheets maintained their curative properties, but also shows that xenogeneic transplantation with human stem cells can be performed and by consequence, allogeneic transplantation is possible [65]. Because cell sheet therapies are a recent medical treatment, we do not have long-term experience on the potential harm of cryopreserved cell sheets after transplantation, and further studies are required to improve new cryopreservation and thawing protocols [66][67]. In the field of cell sheet cryopreservation, very few publications and data are available [65][68][69][70][71], and additional studies will be needed to improve the methodology and the safety of the cryopreservation
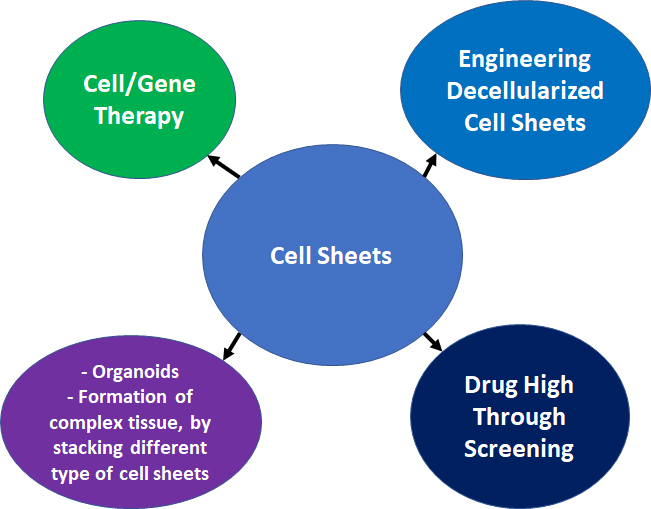
Engineering cell sheet with mesenchymal stem cells is a very important branch in the field of the regenerative medicine, which has been growing for the past 20 years. In addition to the use of the cell sheet for cell therapy, numerous other applications could be used (Figure 1):
Formation of complex tissue, by stacking different type of cell sheets [72];
Gene therapy [73][74][75];
Drug high-throughput screening [76];
Engineering decellularized cell sheets [77][78].
 |
|
Figure 1: Summary of the multiple applications for cell sheets. |