1000/1000
Hot
Most Recent

The silver nanoparticles (AgNPs) have been shown to have bactericidal action. In this study, we demonstrated that ultraviolet A (UVA) irradiation of AgNPs is effective at enhancing their activity. This bactericidal effect is attributable to the UV irradiation-mediated enhanced production of highly reactive hydroxyl radicals generated from AgNPs. The method of UV irradiation is very simple. The UV radiation used in this study was UVA, which is safer for humans than UVC as known to have a strong bactericidal activity. Although challenges persist regarding the effects of AgNPs on human health and the elucidation of the molecular mechanisms underlying the generation of radicals, our findings would contribute to the development of medical materials for further protection against infection.
The AgNPs used in this study were prepared as previously reported [1]. The resulting AgNPs had the same characteristics as those obtained in our previous experiments (maximum absorption at 390 nm, and particle sizes were ~5 nm [2][3][4][5]. To produce UV radiation, we used a universal handy-type UV lamp that was able to generate has a wavelength of 365 nm, putting it in the range of UVA (320 to 400 nm), which is safer than other types of UV radiation (i.e., UVC and UVB). UVB (280 to 320 nm) can cause a variety of damaging effects in various biological cells, whereas UVC (200 to 280 nm) is very hazardous to most organisms [6][7].
We first examined whether hydroxyl radicals are generated when a solution containing AgNPs is subjected to UV irradiation. Hydroxyl radicals were detected when a UV-irradiated solution was subjected to an electron paramagnetic resonance (EPR) analysis (Figure 1A). We measured the amounts of hydroxyl radicals in solutions of AgNPs by varying the time of UV irradiation from 0 to 30 min. The presence of hydroxyl radicals was discernible after adding AgNPs to PBS (“0 min required for UV irradiation” in Figure 1B). We observed UV irradiation-mediated enhanced production of hydroxyl radicals from AgNPs. The proportion of hydroxyl radicals increased as UV irradiation continued for up to 1 min and subsequently plateaued (Figure 1B). The amounts of hydroxyl radicals generated by UV irradiation for 1 and 3 min did not differ significantly. The lifespan of hydroxyl radicals generated was very short, at around 30 s (Figure 1C).
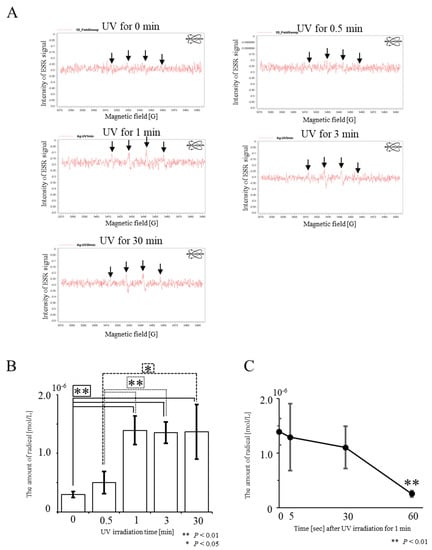
Figure 1. Detection of hydroxyl radicals from UV-irradiated AgNPs using electron paramagnetic resonance (EPR) spectroscopy for the detection of radicals. (A) Raw data obtained from AgNPs UV-irradiated for 0, 0.5, 1, 3, or 30 min. In “UV for 0 min”, a very weak peak for hydroxyl radicals is apparent. Four peaks for hydroxyl radicals, indicated by arrows, are discernible in the UV-irradiation groups. (B) Evaluation of the amount of hydroxy radicals produced after UV irradiation of AgNPs. The amount of hydroxyl radicals was determined from the raw data shown in A. There is no significant difference between “UV for 1 min” and “UV for 3 min”. (C) Time-dependent decrease in the amounts of hydroxyl radicals generated after “UV for 1 min” at 30 s and thereafter the amount of hydroxyl radicals decreases rapidly.
There are several possible mechanisms underlying the generation of hydroxyl radicals induced by UV irradiation of AgNPs. An surface plasmon resonance-mediated collective oscillation of conduction electrons may be generated on the surface of UV-irradiated AgNPs, leading to local generation of an enhanced electric field. Homolytic cleavage, a process in which the covalent bonds of water surrounding AgNPs are broken, may occur in response to the collective oscillation of conduction electrons, resulting in the generation of unpaired electrons as well as radicals. Unfortunately, the current results do not support this hypothesis, and further analysis is required to elucidate the mechanism of action.
Some reports claim that AgNPs yield reactive oxygen species, which act as oxidative stressors to kill various types of bacteria [8][9][10][11]. However, these reports appear to rely on the indirect action of Ag, in which antibacterial activity might be elicited through oxidized peripheral substances or modified materials. Electron paramagnetic resonance spectroscopy is considered one of the standard methods for the quantification of ROS; however, the equipment is very expensive. To our knowledge, there has only been one report indicating that this equipment is useful to detect radicals [12]. In this study, we demonstrated the usefulness of an EPR spectroscopy-based direct detection approach. Our present study differs from the experiments of Zhang et al. [12], who explored the mechanism by which ROS exerts microbicidal activity. We observed a 1.7-fold enhanced production of hydroxyl radicals when AgNPs were irradiated for 0.5 min, and 4.5-fold enhanced production when AgNPs were UV-irradiated for 1 min (Figure 1B). The increased levels of hydroxyl radicals should reflect enhanced microbicidal activity of AgNPs.
We investigated whether UV-irradiated AgNPs inhibit the survival of the bacterium E. coli. Experiments were carried out using two approaches: In the first set of experiments, solutions containing AgNPs were UV-irradiated, and then mixed with E. coli prior to seeding onto agar plates (Figure 2). In the second approach, agar plates that had their surface coated with AgNPs were irradiated with UV and plated with E. coil (Figure 3).
In the first approach, PBS or PBS-containing AgNPs (PBS/AgNPs) was subjected to UV irradiation for 0, 1, or 3 min, after which, each solution was mixed with E. coli prior to plating onto agar plates. After overnight incubation at 37 °C, the number of E. coli colonies growing on plates coated with the AgNPs, which had been UV-irradiated for 1 min (Figure 2A-e,B), was significantly lower than that of untreated E. coli colonies (“UV irradiation for 1 min” in Figure 2A-b,B). Similar results were observed when UV irradiation was performed for 3 min (“UV irradiation for 3 min” in Figure 2A-f,B). The number of E. coli colonies grown in the “AgNPs” group that had not been irradiated was lower than that of those grown in the “PBS” group (PBS vs. AgNPs in Figure 2A-a,d,B). When E. coli were grown on plates coated with PBS, the number of E. coli colonies was not significantly different, even after UV irradiation for 3 min (Figure 2A-a–c,B). These results indicate that the antibacterial effects of AgNPs were significantly enhanced after exposure to UV for 1 min.
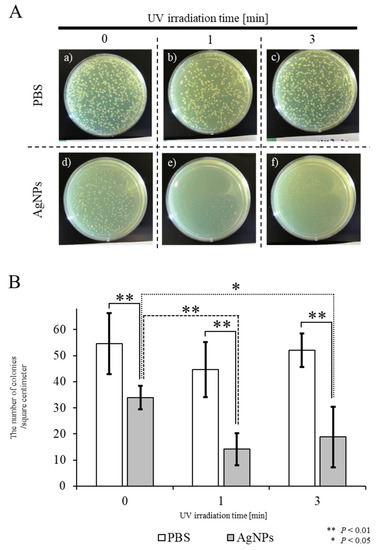
Figure 2. Bactericidal effects of UV-irradiated AgNPs on the survival of E. coli. (A) Appearance of E. coli colonies on agar plates after overnight incubation at 37 °C. Aqueous solutions of PBS or solutions containing AgNPs were subjected to UV irradiation for 0, 1, or 3 min, then each solution was mixed with E. coli prior to plating on agar plates. (B) Quantitation of colony numbers in each group. In the control PBS groups, shown as white columns, there was no difference in the survival of E. coli with or without UV irradiation. In the experimental groups, shown as gray columns, there was a slight decrease in the number of colonies after incubation with non-UV-irradiated AgNPs (designated as “UV for 0 min”). A significant decrease in the number of colonies was observed when E. coli were incubated with AgNPs UV-irradiated for one or three min, designated as “UV for 1 min” and “UV for 3 min”, respectively. Experiments were repeated three times on different days.
In the second approach, in an experimental group designated as “AgNPs/UV”, E. coli were first plated onto agar plate surfaces pre-coated with AgNPs, after which the surfaces were subjected to UV exposure for 1 min (“AgNPs/UV” group). As controls, E. coli were first plated onto agar plate surfaces pre-coated with PBS, after which the surfaces were subjected to UV exposure for 1 min (the “PBS/UV” group) or were not UV-irradiated (the “PBS” group). As an additional control, E. coli were plated onto agar plates pre-coated with AgNPs, which were not subsequently UV-irradiated (“AgNPs” group). In the AgNPs/UV group, the growth of E. coli was suppressed (Figure 3A-a) compared with that of E. coli colonies in the other groups (a vs. c and d in Figure 3A,B). There were statistical differences between the PBS and AgNPs/UV groups (p = 0.0009) and between the PBS/UV and AgNPs/UV groups (p = 0.002) (Figure 3B). Additionally, as in the first experiment, the number of E. coli colonies in the AgNPs was lower than that of “PBS” group (p = 0.009; b vs. d in Figure 3A,B). These results suggest that the generation of radicals from AgNPs is accelerated after UV irradiation, leading to increased toxicity against E. coli. In this study, AgNPs were transiently kept under dry conditions, which was different from the situation in the first approach. As bacteria live in an environment enriched with water, the radicals provided by AgNPs might affect their survival. Alternatively, the survival of bacteria may be influenced by direct contact with AgNPs in the absence of water. In any event, complex materials coated with AgNPs can bring effective bactericidal effects. Our results indicate that UV irradiation of materials coated with AgNPs produces increased antibacterial activity.
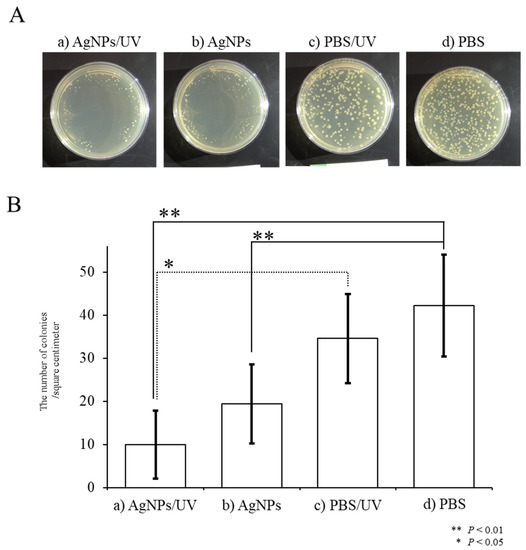
Figure 3. Bactericidal effects of hydroxyl radicals generated in situ on the survival of E. coli. (A) Appearance of E. coli colonies on agar plates after overnight incubation at 37 °C. A solution containing AgNPs was plated onto the surface of agar plates. Then, E. coli were seeded onto the surface of agar plates pre-coated with AgNPs, and the surface was subjected to UV exposure for one min (designated as “AgNPs/UV”) or were not irradiated (designated as “AgNPs”). Alternatively, E. coli were plated onto non-coated agar plates, and then the surfaces were either subjected to UV exposure for one min (designated as “PBS/UV”) or were not irradiated (designated as “PBS”). (B) Quantitation of colony numbers in each group. The presence of AgNPs reduced the number of E. coli colonies. The growth of E. coli was significantly suppressed when a plate was UV-irradiated for one min. Experiments were repeated three times on different days.
The UV radiation used in this study was produced by a universal handy-type UV lamp capable of generating UV of 365 nm, which is within the range of UVA (320 to 400 nm) and is safer than other types of rays such as UVC (200 to 280 nm). UVC is highly biotoxic, in addition to its strong bactericidal action. In contrast, UVA is not only known to be involved in reducing skin elasticity and promoting aging, but also facilitates the exchange of intracellular substances, leading to promotion of cell’s metabolism. Ninety-nine percent of UV rays reaching the earth’s surface from the sun are UVA. This is advantageous, especially when people wearing AgNP-coated protective clothing and medical masks continue to work outside without removing the clothes or masks, because their sterility can be maintained. This UV-enhanced AgNP-based microbicidal system is also useful for protecting against infection in case of occasional unintended contact when healthcare workers change clothes [13]. For the practical use of AgNPs, biosafety issues and environmental safety concerning AgNPs have been a subject for discussion. Hydroxyl radicals, renowned as powerful oxidizing agents, are likely to be effective against viruses, bacteria, and in the degradation of chemicals. Therefore, our system may be valuable for the protection of healthcare workers from infection with pathogens such as COVID-19 and SARS, and in the prevention of outbreaks of similar diseases.