1000/1000
Hot
Most Recent

Heart failure (HF) is one of the major causes of morbidity and mortality worldwide and represents an escalating problem for healthcare system. Therefore, it would be of utmost importance to identify asymptomatic individuals with left ventricular dysfunction before the onset of symptoms. Furthermore, special attention should be focused on individuals who are already classified as NIHA I and "apparently healed" patients, who have been diagnosed with HF and whose clinical condition is stable thanks to therapy. These patients usually suffer from a worsening of their condition over time, and therefore recognizing these changes at the onset would be a great achievement.
Despite the progress made in the field of cardiology in terms of treatment and personalized therapy, heart failure (HF) remains one of the leading causes of morbidity and mortality [1]. Unfortunately, the diagnosis of HF in asymptomatic subjects is challenging and therefore delayed until overt HF [2].
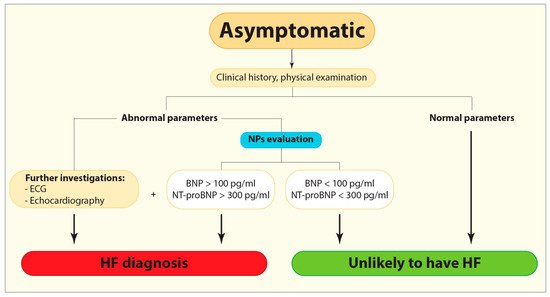
Figure 1. Flow diagram demonstrating the current diagnostic algorithm for HF identification. BNP, B-type or brain natriuretic peptide; ECG, Electrocardiogram; HF, Heart failure; NPs, Natriuretic peptides; NT-proBNP, N-terminal pro-B-type natriuretic peptide.
Cardiac troponin is the gold biomarker for diagnosis of acute myocardial infarction (AMI) and its elevated levels are present in patients with acute and chronic HF as well, indicating the ongoing myocardial damage [9][11]. Troponin levels may be elevated regardless of the ischemic etiology of myocardial injury due to different causes such as LV hypertrophy, hypertension, diabetes mellitus, metabolic syndromes, hypercholesterolemia, and genetics, so its detection in the general population has aroused interest in this marker as an indicator of myocardial injury in asymptomatic individuals [12][13][14]. However, defining the cut-offs among asymptomatic individuals might be challenging since different factors significantly affect troponin levels such as age, gender, body mass index, systolic pressure, diabetes mellitus, severe pulmonary infections and renal failure [12][13][15][16].
Emerging biomarkers such as soluble suppression of tumorigenicity 2 (sST2), galectin-3 (Gal-3) and ghrelin reflect different pathophysiological processes closely associated with fibrosis [12]. Even though the diagnostic potential of these relatively new biomarkers is still lower compared to NPs, their prognostic power proved to be very useful during patient’s follow-up. The American College of Cardiology guidelines, besides a strong recommendation of the use of NPs, has already suggested a multimarker approach since these emerging biomarkers could provide additional diagnostic information to the traditional biomarkers, as well as help regarding risk stratification [8].
Elevated levels of Gal-3 have been detected in patients with LV remodeling and diastolic dysfunction [20]. Huttin et al. demonstrated a significant association between Gal-3 and fibrosis at a very early stage of cardiac changes [21]. However, the use of Gal-3 as a biomarker for asymptomatic LV dysfunction is still debated, mainly due to its low specificity and the fact that its concentration levels could be altered due to inflammation and fibrotic processes in organs other than the heart [22].
Finally, ghrelin, primarily recognized as a gastric peptide, is expressed in the heart, although at a much lower level than in the gastrointestinal tract [23][24]. Given its cardioprotective role against apoptosis and myocardial fibrosis [24], ghrelin and its receptor have become the subject of intensive studies. It has been noted that the interaction between ghrelin and its receptor differs between the early and late phases of HF [24] and that changes in the ghrelin myocardial axis could be detectable even before overt changes in LV function [23]. In our previous study, we observed lower levels of ghrelin in patients with DCM in comparison with healthy controls [24]. Interestingly, among DCM cohort, early diagnosed patients had higher ghrelin levels than patients with a longer duration of the disease [24].
Promising biomarkers that pave their pathways in the cardiovascular fields are microRNA (miRNA), long non-coding RNAs (lncRNA) and exosomes. These molecules are able to give us an insight into various processes involved in HF development such as myocyte loss, hypertrophy, fibrosis and changes in the extracellular matrix that are involved in cardiac remodeling. miRNAs involved in coronary artery disease (CAD) (e.g., miR-624, miR-340, miR-15-5p, miR-21-5p, miR-210-5p, miR-29b-3p, miR-7-5p, miR-99a-5p) [26][27], diabetes (e.g., miR-21) [28], and hyperlipidemia (e.g., miR-122, miR-370) [29][30] could be used as biomarkers since these conditions increase the likelihood of HF development overt time. In the same context, being associated with a higher incidence of diabetes, antisense lncRNA such as CTBP1-AS2 and VIM-AS1 have the potential as prognostic and diagnostic markers as well[31][32].
β secretase-1 antisense (BACE1-AS) lncRNA and exosomes cargo, consisting of exo-miRNA-192, exo-miRNA-194, and exo-miRNA-134a might be indicators of cardiomyocyte death[33], whereas CTBP1-AS2 and VIM-AS1 mediate cardiomyocyte hypertrophy and fibrosis respectively [34][35]. In addition, exosome cargo consisting of exo-miRNA-21-3p, exo-miRNA-132, and exo-miRNA-200 [36] and non-coding RNAs such as miR-1, miR-133a indicate the early phases of hypertrophy and could be very helpful in its timely identification. [37].
Early recognition of asymptomatic individuals prior to overt HF development would reduce morbidity and mortality associated with the disease. Traditional and emerging biomarkers are reserved for the symptomatic phase, therefore there is a need for further studies regarding their practical use in asymptomatic settings. Non-coding RNA and exosomes cargo, by the same token, may provide an insight into undergoing processes that could lead to HF over time and therefore could be very useful as prognostic biomarkers. Given the fact that different biomarkers are involved in multiple harmful pathophysiological processes associated with HF, the multimarker approach might enable the early identification of patients at risk of HF.