1. Cryotropic Gelation
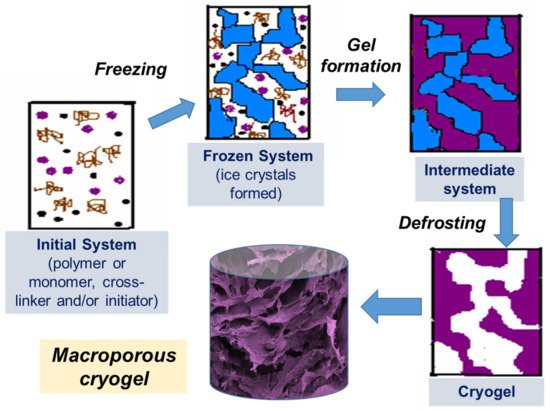
Cryotropic gelation is the formation of a hydrogel under semi-frozen conditions, when most of the solvent crystallizes, and gelation occurs in small non-frozen areas around the solvent crystals (
Figure 1)
[1][2][3]. The solvent crystals perform as a template for the pores, and after melting, they leave large voids filled with liquid solvent
[2]. The most common solvent used is water, but other solvents or solvent mixtures with freezing points reasonably close to zero can be used, such as dimethyl sulfoxide (DMSO), benzene or cyclohexane
[4][5]. Water is considered to be the best solvent for the formation of biocompatible cryogels for biomedical applications. Gelation can occur through different mechanisms, usually through the formation of covalent bonds, or physical interactions (ionic bonding, hydrogen bonding and others)
[6]. Synthesis by radical polymerization is the most commonly used method of preparing polymer cryogels. Acrylate derivatives are mostly used as gel-forming units together with an ammonium persulphate and tetramethylethylenediamine (APS/TEMED) initiating system in these reactions. Cryogels based on natural polymers can be prepared by cross-linking intact or pre-modified polymers utilizing the amino, carboxyl, hydroxyl and other functional groups. Variation of the cryotropic gelation parameters, such as the freezing temperature, cooling rate, and the presence of the ions or other solutes, as well as the polymer and solvent content, allows the tuning of cryogel properties for a specific application.
Figure 1. Scheme of preparation of cryogels: (i) initial system (the gel precursor solution is cooled down to a temperature below freezing point); (ii) frozen system (the ice crystals formed and solutes are concentrated in non-frozen zones); (iii) intermediate system (gel is formed in non-frozen liquid around ice crystals); (iv) cryogel is formed upon defrosting.
2. Cross-Linking
Cross-linking is one of the major factors that affect the mechanical and biological properties of cryogel scaffolds and their biodegradability. The types of cross-linking are summarized in
Table 1. Although cryogel scaffolds can be readily formed through physical cross-linking—for example, involving ionic interaction and hydrogen bonding—they are generally reversible, and the resultant porosity and mechanical properties will not always be satisfactory for all applications. Poly(vinyl alcohol) (PVA) is one of the most studied synthetic polymers that can be physically cross-linked to obtain cryogels
[1][7]. PVA cryogels are formed as a result of the formation of microcrystalline zones and are thermally reversible. The pore size of PVA cryogels is less than 10 µm. They have been used in a number of biotechnological applications for the immobilization of enzymes
[8] and cells
[9], as well as in tissue engineering, mainly for mimicking bone and cartilage tissues
[10][11].
Table 1. Types of the cross-linking reactions for the formation of cryogel.
| Cross-Linking Type |
Methods |
Examples |
References |
| Physical cross-linking |
Ionic interaction |
Alginate cryogel cross-linked with Ca2+ |
[12] |
| Ionic cryotropic gelation of pectin-chitosan |
[13] |
| Freezing-thawing cycle |
PVA-gelatin cryogel; hydrogen bonding, formation of crystallites of PVA |
[14] |
| Agarose cryogel, intramolecular and intermolecular hydrogen bonding |
[15] |
| Chitosan cryogel, formation of crystallites composed of D-glucosamine and N-acetylglucosamine units |
[16][17] |
| Chemical cross-linking |
Reaction between the functional groups of polymer and cross-linker or polymer–polymer |
Condensation of an amine with the carbonyl group, formation of Schiff
bases; chitosan and gelatin cross-linked with glutaraldehyde. |
[18][19][20][21][22] |
HEMA-Gelatin: radical polymerization and cross-linking with
poly(ethylene glycol) diacrylate (PEGDA) |
[23] |
Reactions of multifunctional-amine and epoxy groups; chitosan and
diglycidyl ethers of ethylene glycol (EGDGE) or polyethylene glycol (PEGDGE) |
[24] |
Reaction between amine and carboxy groups: gelatin, gelatin-heparin
cross-linked with EDC/NHS |
[25][26] |
Oxime-cross-linking of gelatin-oxyamine and hyaluronan-aldehyde;
gelatin-hyaluronan cryogel |
[27] |
Radiation cross-
linking, exposure to high energy source (electron beam) |
Electron-beam initiated cross-linking reaction; dextran, hyaluronan |
[28][29][30] |
| Photo-cross-linking |
UV photo-cross-linking; hyaluronic acid, dextran |
[31][32] |
An example of non-covalent ionic-type cross-linking is the preparation of ionic cryogels as a result of the growth of metal-polymer-coordinated complexes and electrostatic interactions between oppositely charged groups of chitosan and metal ions such as PdCl
42−, PtBr
4 2− and PtCl
42− at sub-zero temperatures
[33][34]. Alginate-based cryogel can be obtained by introducing ionic links between anionic polysaccharide macromers with divalent cations such as Ca
2+ [12].
Self-assembly of peptides is another example of the formation of non-covalent cross-linked cryogels. Peptide cryogels were obtained by pH-sensitive self-assembly of fluorenyl-9-methoxycarbonyl (Fmoc)-diphenylalanine (Phe-Phe) into fibers in an apparently frozen system
[35]. Fmoc-Phe-Phe-derived cryogels were noticeably less mechanically strong than equivalent hydrogels prepared from the same concentration of Fmoc-Phe-Phe at ambient temperature. This could be due to the effect of cryostructuration: pre-concentration of Fmoc-Phe-Phe at low temperatures leading to much higher concentrations of Fmoc-Phe-Phe compared to the bulk concentration in the hydrogel precursor solution, which may affect the formation of the fibers. In addition, the formation of large pores creates a more heterogeneous structure in cryogels than in hydrogels, which can reduce the mechanical stability of the material
[35]. Usually, cryogels obtained by radical polymerization or covalent cross-linking are mechanically stronger than hydrogels obtained from the same gel precursor solution at room temperature. In the case of Fmoc-Phe-Phe-derived cryogels, the 3D structure was supported by weaker non-covalent interactions, which may explain why cryogels were mechanically less strong.
Covalent cross-linkers are selected based on the chemistry of the polymer used. Bifunctional acrylate derivatives, such as
N,
N′-methylenebisacrylamide (MBA), poly(ethylene glycol) diacrylate (PEGDA) and others have been used to provide additional connections between formed polymer chains during radical polymerization. A range of different cross-linkers are used for cross-linking biological polymers. 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC), alone
[36][37] or in combination with N-hydroxysuccinimide (NHS)
[38][39], is an important water-soluble activator for carboxyl and phosphate groups of biopolymers that makes them reactive, respectively, toward amino and hydroxyl groups. EDC/NHS generates peptide-like bonds and is well used as a potentially biocompatible cross-linker in the biomaterial fields. EDC is limited to cross-link molecules that are directly adjacent to each other, whereas glutaraldehyde (GA) provides more flexible cross-linking with longer linkage between molecules.
GA has been widely used as a cross-linking agent for collagen, gelatin, chitosan and other polymers due to its low cost, high solubility and stability in aqueous solutions
[40][41]. However, the excessive incorporation of GA into scaffolds can have implications for biocompatibility. Some adverse effects of GA modifier, such as the inhibition of cell proliferation, have been reported, and it was suggested that the lowest concentration that does not compromise the mechanical properties of the cryogel should be used
[42]. Additionally, to improve biocompatibility of the scaffolds cross-linked with GA, residual aldehyde groups and formed Schiff’s base in the cryogel have to be reduced by sodium borohydride
[18][43]. The removal of toxic sodium borohydride itself requires extensive washing of the material, which can be time-consuming and expensive. Alternatively, a hydrophilic polysaccharide dextran pre-modified with aldehyde groups was used as a cross-linker for gelatin and chitosan
[44][45]. The viability of mouse embryo NIH 3T3 fibroblasts was considerably higher (∼80%) on modified dextran cross-linked scaffolds than on scaffold cross-linked with GA (40%)
[44], which indicates a lower toxicity of the modified dextran cross-linked scaffolds.
Genipin, a natural product extracted from the gardenia fruit, has been utilized as a biocompatible cross-linker to obtain hyaluronic acid (HA) based cryogels
[46]. The effect of GA, genipin, and EDC/NHS on the formation of carrageenan-alginate scaffolds by freeze-drying was analyzed
[38]. Scaffolds produced using EDC/NHS cross-linking resulted in better cellular response and higher metabolic activity, as well as demonstrating biocompatibility properties, and being porous and physically and mechanically stable. Thus, even where genipin has been proposed as a non-toxic cross-linker, the final choice of cross-linker must be determined by the application and properties of the scaffold, and an alternative cross-linkers must be taken into consideration in order to achieve best results. Another limitation of genipin is that cross-linking produces a material that has a strong blue color, which is not always desirable.
Kirsebom et al. studied the cryotropic gelation of gelatin and casein catalyzed by transglutaminase (TGase) enzyme. TGase catalyzes the linkage of the γ-carboxyamide group of glutamine and the ε-amino group of lysine
[47]. The enzymatic cross-linking under partly frozen conditions was a slow process, requiring at least 14 days to form gels that were sufficiently stable to handle. The gels, however, were not formed at room temperature, indicating the role of freezing in the gelation process. The mechanical properties of gelatin and casein cryogels improved with increasing protein content
[47]. There is some potential advantage to enzymatic cross-linking, such as mild reaction conditions and no need to use other chemicals as cross-linking agent. However, the enzyme activity under cryogenic conditions is low and cryogel formation takes a long time.
The use of external energy sources, such as UV radiation, is another alternative in the synthesis of cryogels which does not use chemical initiators. Cryogels from mixed semi-dilute solutions of 2-hydroxyethylcellulose and chitosan have been obtained using UV radiation with H
2O
2 as a photoinitiator
[48]. The advantages of this process are the relatively short time required for cryogel formation and the fact that no additional purification is needed. Unfortunately, this method is limited by the low penetration depth of photons. Another approach to cross-linking was used in
[28][29][30], where polymerization of acrylated dextran and hyaluronan was initiated by accelerated electrons (an E-beam). This method uses no toxic initiators, and results in sterile, elastic scaffolds with a controlled pore size, excellent swelling and low flow-resistance properties
[30]. The reaction time was short—less than 10 min—and with double-sided irradiation, cryogels up to 7 cm thick could be obtained. Some degradation occurred under irradiation along with cross-linking, and the degradation products were washed out, resulting in 80% of the hyaluronan being incorporated into the scaffold
[28]. Even though this is a simple cross-linking process, it requires special electron beam equipment and the modification of polysaccharides with acrylate groups, followed by extensive purification before polymerization. Another disadvantage is the limitation of the cryogel size that can be produced and the impossibility of using it to obtain larger samples.
4. Effect of Freezing Conditions
The freezing temperature affects the pore size and wall thickness of cryogels
[19][49]. The highest freezing temperature and the slowest cooling rate produces the biggest pores in cryogels and lower temperature and faster freezing rates are required to produce the smallest pores. The reason for this is the different rates of growth of solvent crystals. Bigger solvent crystals form at slower cooling rates, and this creates bigger pores. At lower temperatures, more solvent will freeze, creating less space between the solvent crystals, where the gel precursors will concentrate forming denser polymer walls. At lower temperatures, the rate of nucleation is higher, which leads to the formation of more crystals of smaller size, thus creating smaller pores in the final cryogel material
[19].
In general, the solvent crystals must form before gelation has finished. When cryogels are synthesized by radical polymerization, a lower concentration of initiator is usually used to slow down the polymerization rate and produce cryogels with large pores
[51]. If the gelation occurs faster than the ice crystal formation, large, interconnected pores will not form and the cryogel will have a morphology similar to a hydrogel obtained at room temperature. A gel formed under these conditions will have less porosity, less mechanical strength, and will be brittle in nature
[50]. Hixon et al. compared cryogel scaffolds with the conventional hydrogel-based scaffolds of three different materials (chitosan-gelatin, N-vinyl-2-pyrrolidone, and silk fibroin (SF)) to identify the optimal material and form of scaffold for use as a graft substitute in bone regeneration
[52]. Cryogels proved superior to hydrogels in terms of swelling potential, porosity and mechanical properties, regardless of the polymer used
[52]. The microstructure of the cryogels depended on the kinetics of the gelation and nucleation and could be controlled, as mentioned above, by temperature as well as by the concentrations of the initiator or cross-linker
[51].
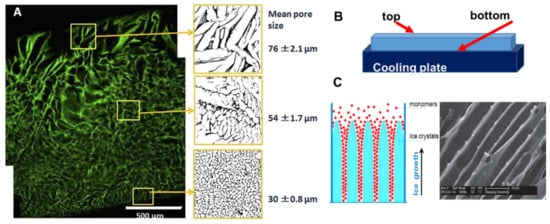
A heterogeneous structure of the cryogels forms under unidirectional freezing. This was observed in the preparation of sheets of cryogels, where the pore size varied from top to bottom. The cryogel had smaller pores at the bottom (in contact with the freezing plate) and the pore size gradually increased towards the top (
Figure 2A)
[20]. This anisotropic morphology can be beneficial for wound healing because it creates a special environment for cells and controls their migration. When material is applied with smaller pores at the top and larger pores at the wound bed, it stimulates cell migration from the wound into the material. An additional benefit of placing larger pores of the material on the wound bed is increased flexibility to adjust to the roughness and curvature of the wound surface
[20].
Figure 2. Anisotropic structure in cryogels: (
A) gelatin cryogel frozen in a petri dish, (
B) scheme of set up for unidirectional freezing, and (
C) the scheme of ice-crystals grows and scanning electron microscopy image of PEG-based cryogel. (Figure (
A) is adapted from
[2] and used under a Creative Commons Attribution 4.0 International License and (
C) is adapted from
[53] with permission from The Royal Society of Chemistry.
Many natural tissues, such as those in nerves, cartilage, ligaments, tendons, and the spinal cord, have oriented structures. The morphology of tissue scaffolds should mimic these natural tissues, and cryogels with an aligned porous structure are preferable for better physiological and mechanical function of the tissue scaffold. Unidirectional freezing has been used to create aligned porous structures in both synthetic and natural polymer cryogels
[53][54]. During unidirectional freezing, ice crystals form and grow in one direction, forming an aligned porous structure after melting (
Figure 2C). Poly(ethylene glycol) cryogels were prepared using PEGDA of various molecular weights: 200, 700 and 2000 Da as a precursor
[53]. Cryogels with a smaller pore diameter were obtained using lower molecular weight 200 Da, PEGDA-200, while larger pores were observed when PEGDA with a molecular weight of 2000 Da was used (PEGDA-2000). The smaller pores of the PEGDA-200 cryogel were observed because PEGDA-200 exhibited a marked tendency to adsorb on the surface of the ice crystal during freezing, inhibiting crystal growth. Smaller ice crystals resulted in smaller pore diameters
[53]. Freezing temperature was an important parameter for unidirectional freezing because it influenced the rate of formation, size and orientation of solvent crystals. Using a higher temperature leads to the formation of cryogels with large pore diameters. In the work by Wu et al., frozen acetic ether, frozen ethyl alcohol and liquid nitrogen were used to achieve freezing temperatures of −80 °C, −110 °C and −196 °C, respectively. The resulting cryogels had anisotropic compressive strength in accordance with the directions of the pores, and the diameter of the microtubule channels could be controlled in the range 10 to 50 μm by adjusting the molecular weight of the PEGDA and the freezing temperature
[53]. Using the same approach, a chitosan-gelatin cryogel with aligned porous structure was prepared inside a polyurethane tube for peripheral nerve regeneration
[54].