1000/1000
Hot
Most Recent

Diabetic patients often present diabetic kidney disease (DKD), a burdensome complication that can be silent for years. The average time of onset of kidney impairment in diabetic patients is about 7–10 years. The clinical impact of DKD is dangerous not only for the risk of progression to end-stage renal disease and therefore to renal replacement therapies, but also because of the associated increase in cardiovascular events.
Diabetes mellitus (DM) is the leading cause of kidney failure globally [1]. Specifically, diabetic kidney disease (DKD), which is defined as elevated urine albumin excretion or reduced glomerular filtration rate (GFR) or both, is a serious complication that occurs in up to 40% of all diabetic patients [2].
The clinical and socio-economic impact of DKD is burdensome not only because of the risk of progression to end-stage renal disease (ESRD) and therefore to renal replacement therapies, but also because of the associated increase in cardiovascular (CV) risk [3][4]. A strict control of blood glucose is essential in DKD. Although many antidiabetic agents are currently available, the treatment of diabetes in DKD is challenging. Many antidiabetic drugs are contraindicated in advanced CKD, and others require dose adjustments due to an increased risk of drug toxicity as a result of reduced renal excretion [5][6].
Both genetic and environmental variabilities represent risk factors of disease progression. Besides the non-modifiable risk factors, such as family history, genetics, gender, age at diagnosis, and DM duration, lifestyle can be improved promoting healthy habits. It is important to maintain a proper glycemic control, blood pressure, avoid or quit smoking, reduce alcohol consumption, practice physical activity, follow a balanced diet and maintain a healthy lipidic profile [7].
It is of paramount importance to guarantee a structured education for patients and health care professionals to raise awareness to the role of DM and DKD prevention. Self-management knowledge should be used as an adjunct therapeutic option, especially in high-risk patients.
The pathophysiology of DKD is multifactorial and characterized by a critical metabolic impairment; the upstream influence of hyperglycemia leads to a dysregulated intracellular metabolism, inflammatory lesions, increased apoptosis processes and tissue fibrosis [8]. At the basis of DKD injury there are three crucial steps: (1) glomerular hypertrophy leading to hyperfiltration. Glomerular hyperfiltration is present in up to 75% of T1DM patients and up to 40% of patients with T2DM and is a typical feature of early DKD manifestations [9]; (2) glomerular and tubulointerstitial inflammation, related to chemokines, cytokines, and profibrotic factors activation; (3) dysregulated cellular apoptosis and changes in the extracellular matrix. These mechanisms lead to glomerular basement membrane thickening, podocyte depletion, mesangial matrix expansion, and tubular damage. All these factors may contribute to the progression of DKD, resulting in vascular remodeling, endothelial dysfunction, glomerulosclerosis, and tubulointerstitial fibrosis [10][11][12].
Different intracellular pathways demonstrated a driving role in the DKD process, stimulated by hyperglycemia. High blood glucose stimulates protein kinase C beta type (PKC-beta) and protein kinase C delta type (PKC-delta) activation in the renal cortex. This mechanism triggers the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and the release of both interleukin (IL)-6 and the tumor necrosis factor (TNF)-α by endothelial and mesangial cells [13][14]. The advanced glycation end-products species (AGEs) pathway not only alters the reactive oxygen homeostasis in a pro-oxidant way [15][16] but also contributes to the ultrastructural changes of the mesangial matrix, with a preferential localization to nodular lesions of DKD patients [17].
In addition to PKC and AGEs-guided mechanisms, more intracellular pathways seem to be implicated in the DKD insult. NF-κB, inducible nitric oxide synthase, JAK/STAT, and transforming growth factor-beta1/SMAD pathways are all leading to the production of proinflammatory molecules inducing extracellular matrix deposition and the differentiation/proliferation of myofibroblast in DKD patients [18][19][20][21].
A timely recognition of the risk factors for DKD progression can be crucial in decreasing morbidity and mortality in diabetic patients.
Several wake-up calls should alarm diabetic patients regarding their kidneys’ health, and patients should be referred to a nephrologist earlier if they present rapid renal reduction, resistant hypertension, hyperkalemia, UACR exceeding 300 mg/g, or other urinary abnormalities [22].
A proper remodeling of lowering glucose therapy is one of the main points that should be evaluated in the evolution from DKD to ESRD. Diabetic patients with ESRD present high levels of blood urea nitrogen, leading to carbamylated hemoglobin production; these molecules are not distinguishable from glycosylated hemoglobin by electrophoresis, causing incorrect elevated levels of hemoglobin A1C [23]. Moreover, the reduced lifespan of red blood cells, iron deficiency, and erythropoietin-stimulating agents can lead to an undervaluation of glucose control [24].
Most oral diabetes drugs are contraindicated in ESRD and the pharmacological therapy should be balanced to avoid over- and undertreatment.
For DKD patients, the transitional ambulatory can represent an opportunity to be evaluated also for non-pharmacological treatments. Renal pre-emptive transplantation or combined pancreas-renal transplantation can represent a suitable option for selected subjects, especially for T1DM patients. Despite the significant improvement in DKD treatment in the last decades, these patients remain at higher risk of ESRD development and mortality; a pre-emptive transplant can strongly improve their quality of life and life expectancy [25].
DKD is a crucial harm in patients affected by DM because it represents a risk of CKD progression up to ESRD and increased CV morbidity and mortality. DKD treatment addresses both problems with first-choice drugs represented by renin-angiotensin system (RAS) blockade, including either angiotensin-converting enzyme inhibitors (ACEi) or angiotensin II receptor blockers (ARB). These drugs played a pivotal role in reducing albuminuria and slowing GFR losses in several clinical trials, such as the Collaborative study (captopril) [26], RENAAL (losartan) [27], and the IRMA and IDNT studies (irbesartan) [28][29].
Particular attention should be paid to transient changes in the serum levels of potassium and creatinine after RAS blockade introduction. A dual blockade with ACEi/ARB or their association with either mineralocorticoid receptor antagonists (MRA) or a renin inhibitor is also discouraged.
Due to the reduced renal excretion, many antidiabetic drugs (substantially excreted via the kidney) are contraindicated or require dose adjustments in DKD patients to prevent hypoglycemia [30][31][32] (Table 1). Metformin has been shown to be safe and effective in glycemic control in patients with T2DM, but it is contraindicated if GFR <30 mL/min/1.73 m2; SGLT2i, on the other hand, have low hypoglycemic effect in patients with impaired renal function, and therefore their use should be restricted in such patients [30][31].
| Drug Class | Medications | Recommendation |
|---|---|---|
| Biguanides | Metformin | Contraindicated if GFR <30 mL/min/1.73 m2 Not started in GFR 30–45 mL/min/1.73 m2 |
| SGLT2 inhibitors | Empagliflozin | Avoid use or discontinue if GFR <45 mL/min/1.73 m2 |
| Canagliflozin | Avoid use if GFR <30 mL/min/1.73 m2 Dose adjustment in GFR 30–59 mL/min/1.73 m2 |
|
| Dapagliflozin | Contraindicated if GFR <30 mL/min/1.73 m2 Not started in GFR 30–45 mL/min/1.73 m2 |
|
| First-generation sulfonylureas | Acetohexamide, tolazamide, tolbutamide, chlorpropamide | Avoid use |
| Second-generation sulfonylureas | Glyburide | Avoid use |
| Glimepiride | Start cautiously in GFR <15 mL/min/1.73 m2 | |
| Glipizide | No dose adjustment | |
| Glicazide | No dose adjustment | |
| Alpha-glucosidase inhibitors | Acarbose | Contraindicated if GFR <30 mL/min/1.73 m2 |
| GPL-1 receptor agonists | Exenatide | Contraindicated if GFR <30 mL/min/1.73 m2 |
| Lixisenatide | Contraindicated if GFR <15 mL/min/1.73 m2 | |
| Liraglutide | No dose adjustment | |
| Albiglutide | No dose adjustment | |
| Dulaglutide | No dose adjustment | |
| Thiazolidinediones | Pioglitazone | No dose adjustment |
| Rosiglitazone | No dose adjustment | |
| Meglitinides | Repaglinide | Start cautiously in GFR <15 mL/min/1.73 m2 |
| DPP-4 inhibitors | Sitagliptin | Lower dosage |
| Vildagliptin | Lower dosage | |
| Saxagliptin | Lower dosage | |
| Alogliptin | Lower dosage | |
| Linagliptin | No dose adjustment | |
| Insulins | Dose adjustment based on patient response | |
| Statins | Normal to Mildly Decreased (GFR: ≥90 to 60–89 mL/min/1.73 m2) |
Mildly/Moderate Decreased to Kidney Failure (GFR: 45–59 to <15 mL/min/1.73 m2) |
|---|---|---|
| Lovastatin | No dose adjustment | NA |
| Fluvastatin | No dose adjustment | 80 mg/day |
| Atorvastatin | No dose adjustment | 20 mg/day |
| Rosuvastatin | No dose adjustment | 10 mg/day |
| Simvastatin/Ezetmibe | No dose adjustment | 20 mg/day |
| Pravastatin | No dose adjustment | 40 mg/day |
| Simvastatin | No dose adjustment | 40 mg/day |
| Pitavastatin | No dose adjustment | 2 mg/day |
Glycemic control in DKD patients is strongly recommended not only for cardiovascular prevention, but also to prevent DKD progression [36]. Glycemic management in patients affected by DKD is challenging due to several factors, such as therapeutic inertia, monitoring difficulties, and the complexity regarding the use of the available treatments [37]. Indeed, one of the main issues in glycemic control in DKD patients is that the risk of hypoglycemia increases with a decreasing GFR, mainly because of the altered pharmacodynamic and pharmacokinetic profiles of antidiabetic drugs and the reduced kidney mass [38].
Along with glycemic control, the control of blood pressure and blood cholesterol levels is crucial to slow DKD progression and prevent its macrovascular and microvascular complications [39][40].
Due to their complex clinical conditions, DKD patients generally take many drugs to slow the progression of their renal disease, prevent specific complications, and manage comorbidities [41], thus leading to an increased risk of experiencing adverse drug reactions (ADRs) and drug-drug interactions. Moreover, the worsening of renal function is often caused by the use of nephrotoxic drugs, especially when used for a long period and at high dosages [42]. All these factors make appropriate drug prescribing more challenging in such a population of patients.
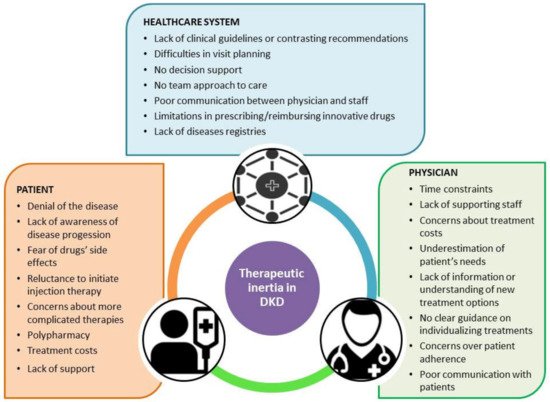
(A) Patient level: Diabetic patients should be conscious of the care plans and target value for the best DKD management: glucose, creatinine, GFR, blood urea nitrogen, phosphorus, calcium, PTH, Vitamin D, albumin, lipid, potassium, and hemoglobin targets. A proper management of blood pressure control and pulse pressure targets is essential. The patient should be motivated to follow a balanced dietary intake and know the best nutrients to choose to reach the desirable glucose values.
(B) Clinician level: High-quality diabetes care requires creating a multi-specialist team that can gain a complete vision of the patient’s status and study the best strategies for implementing cures. Bridging fundamental approaches to care optimization for general practitioners, diabetologists, dieticians, nephrologists, and pharmacologists is critical. The team must perform a “treat to success” management approach rather than a “treat to failure” strategy [44]. Specialists and general practitioners should co-work to make the patient conscious of the importance of a proper glycemic and pressure control. An adequate doctor-patient communication should be promoted. The team must constantly ensure that the patient fully understands the therapeutic modifications and his health status variations. Psychological help should be guaranteed by professionals, especially to treat depression-related symptoms or to gradually overcome the denial of the disease.