Nowadays, consumers have become more conscious about food ingredients, which has led to a growing demand for healthy natural products, and reinforced microalgae as an emerging and rich source of nutrients to be used in food supplementation
[1]. There has been an increasing interest in ω3 long-chain (LC) polyunsaturated fatty acids (PUFAs) for nutritional and pharmaceutical applications. The nutritional importance of ω3 LC-PUFAs, mainly eicosapentaenoic acid (EPA, 20:5 ω3) and docosahexaenoic acid (DHA, 22:6 ω3), for human health is well established. Nevertheless, since humans cannot synthesize, in adequate levels, fatty acids with more than 18 carbons, they must be obtained from seafood, which is the major source of LC-PUFAs, particularly EPA and DHA
[2]. Several studies have shown that EPA and DHA play an important role in the functional growth of brain cells, in preventing/reducing cardiovascular and inflammatory diseases, and also in preventing the progression of some types of cancer
[1][2][3][4][5]. DHA is the predominant synaptosomal plasma membrane LC-PUFA in the brain, important for the normal neurological development. DHA has also been associated with positive effects on memory-related learning ability in Alzheimer’s disease
[4][6].
LC-PUFAs constitute a large share of marine algal lipids, with planktonic algae being the source of most ω3 FAs in fish
[7]. There has been an increasing interest in microalgal lipids mainly because of their ability to synthesize high quantities of LC-PUFAs, as they are in fact the primary producers of ω3 LC-PUFAs, since they contain the necessary enzymes
[8]. Microalgal lipids are divided into neutral lipids (triacylglycerols, diacylglycerols, and sterol esters), mainly located in lipid droplets in the cytoplasm or plastids, and polar lipids (phospho- and glycolipids), which build the fabric of cellular membranes
[8]. The studied marine microalga
Isochrysis galbana is a highly valuable source of natural bioactive compounds with important biological activities, such as hypocholesterolemic action
[9]. The biomass of
I. galbana is promising as a functional ingredient due to its considerably lipid content (20–30% dw) and richness of ω3 LC-PUFAs (mainly EPA and DHA)
[2][10]. In addition, this marine microalga can provide highly valuable biological compounds, such as sterols, tocopherols, and fucoxanthin
[2][9][11].
The change in dietary patterns in the human population, which has been particularly intense in the Western world, has led to an increase in ω6 FA consumption and a decrease in ω3 FA consumption, thus leading to an imbalance in the ω3/ω6 ratio level (desirably > 1). Very low ω3/ω6 ratios promote cardiovascular and inflammatory and autoimmune diseases, whereas increased levels of ω3 LC-PUFAs exert beneficial effects
[12]. Given this background, efforts have been made to replace part of the vegetable or animal fat with marine lipids in foods such as mayonnaise, milk, bread, salad dressing, spreads, and yogurts
[13]. Most food products have been prepared with fish oil, but, more recently, functional foods with a high content of algal ω3 LC-PUFAs have been tested, thus eliciting an industrial effort to produce such nutraceuticals. Moreover, the market for microalgae-containing foods has been expanding
[14]. For instance, the microalgae
Arthrospira platensis, Chlorella vulgaris, and
I. galbana have been previously added as functional ingredients to biscuits
[11][15], bread
[16], and pasta
[17].
Yogurt, one of the most consumed fermented dairy products in the world, is able to ensure the daily intake of nutrients and to bring positive impacts on consumers’ health due to its active cultures that promote healthy digestion and boost the immune system, providing health benefits
[18]. Therefore, yogurt is an ideal vehicle to incorporate ω3 LC-PUFAs
[19]. Dairy products such as yogurts have shown a high potential as carriers of microalgal biomass, ensuring a high share of microalgae ω3 LC-PUFAs and their bioaccessibility, given yogurt’s chemical and rheological properties, easiness to incorporate emulsions, and oxidative stability
[3][20]. In fact, various yogurt products containing DHA have already been developed and marketed
[19].
The aim of this work was to formulate a high-value functional food by the incorporation of freeze-dried biomass and ethyl acetate lipidic extract of Isochrysis galbana in commercial plain yogurt in order to increase ω3 LC-PUFAs content (mainly DHA) and to enhance ω3 LC-PUFAs bioaccessibility. Thus, based on the study’s results, the formulation of the functional yogurt could be optimized for maximal bioavailability of ω3 LC-PUFAs.
2. Yogurt Enriched with Isochrysis galbana
The microalga incorporation level of 2% (
w/
w) in the solid yogurts was chosen based on the literature available over microalgal biomass incorporation into food products
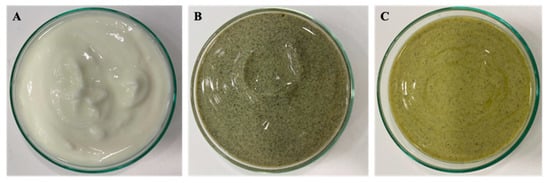
[11][15][17], in order to not compromise the sensory acceptability of the final product in terms of color, fishy flavor and odor. The functional yogurts with 2% (
w/
w) of
I. galbana of freeze-dried biomass and 2% (
w/
w) of
I. galbana ethyl acetate extract incorporation presented an innovative green tonality (
Figure 1).
Figure 1. Yogurt products preparation: (A) Control Yogurt; (B) Yogurt with 2% (w/w) of I. galbana freeze-dried biomass; (C) Yogurt with 2% (w/w) of I. galbana ethyl acetate extract.
The sensory attributes of the novel functional yogurt and the consumer’s acceptance need further evaluation.
2.1. Proximate Composition
The proximate composition of
I. galbana freeze-dried biomass,
I. galbana ethyl acetate lipidic extract, control yogurt and functional yogurts is presented in
Table 1. The moisture content detected in
I. galbana biomass was low (7.6 ± 0.1% dw), which was expected since the studied microalga biomass was freeze-dried. The dry matter of
I. galbana was mainly composed of protein and lipids, 38.7 ± 0.0% dw and 24.5 ± 0.6% dw, respectively. The ash fraction was also a significant share of the biomass (14.6 ± 0.0% dw). The
I. galbana ethyl acetate extract lipid content was 21.4 ± 0.9% dw. The observed proximate composition in the studied microalgal biomass is similar to that reported by other authors
[10][17][21][22][23].
Table 1. Proximate composition (%) of I. galbana freeze-dried biomass, I. galbana ethyl acetate extract, control yogurt, yogurt with 2% (w/w) of I. galbana freeze-dried biomass, and yogurt with 2% (w/w) of I. galbana ethyl acetate extract.
| Proximate Composition |
I. galbana Freeze-Dried Biomass |
I. galbana Ethyl Acetate Extract |
Control Yogurt |
Yogurt with I. galbana Freeze-Dried Biomass |
Yogurt with I. galbana Ethyl Acetate Extract |
| (% Dry Weight) |
(% Dry Weight) |
(% Wet Weight) |
(% Wet Weight) |
(% Wet Weight) |
| Moisture |
7.6 ± 0.1 |
- |
87.9 ± 0.1 a |
86.7 ± 0.0 b |
87.8 ± 0.1 a |
| Ash |
14.6 ± 0.0 |
- |
0.7 ± 0.0 a |
1.0 ± 0.0 b |
0.7 ± 0.0 a |
| Protein |
38.7 ± 0.0 |
- |
3.2 ± 0.1 a |
4.0 ± 0.1 b |
3.2 ± 0.1 a |
| Lipid |
24.5 ± 0.6 |
21.4 ± 0.9 |
2.3 ± 0.3 a |
2.7 ± 0.0 a |
2.6 ± 0.1 a |
2.2. Lipid Classes
The lipid class distribution before and after digestion (bioaccessible fraction) of I. galbana freeze-dried biomass, I. galbana ethyl acetate lipidic extract, control yogurt and functional yogurts is presented in Table 2.
Table 2. Lipid class distribution (% of total lipid) before and after digestion (bioaccessible fraction) of I. galbana freeze-dried biomass, I. galbana ethyl acetate extract, control yogurt, yogurt with 2% (w/w) of I. galbana freeze-dried biomass and yogurt with 2% (w/w) of I. galbana ethyl acetate extract.
| Sample |
|
Lipid Classes |
| TAG 1 |
FFA 2 |
Polar Lipids |
Sterol |
| I. galbana freeze-dried biomass |
Initial |
36.8 ± 3.1 aA |
32.6 ± 1.6 aA |
14.8 ± 2.3 aA |
15.9 ± 0.9 aAB |
| Bioaccessible |
nd bλ |
53.9 ± 4.8 bλ |
22.2 ± 2.6 bλ |
23.9 ± 5.1 aλ |
| I. galbana ethyl acetate extract |
Initial |
18.1 ± 1.1 B |
55.8 ± 0.1 B |
13.7 ± 0.0 A |
12.5 ± 1.2 A |
| Bioaccessible |
- |
- |
- |
- |
| Control Yogurt |
Initial |
47.1 ± 0.2 aC |
25.2 ± 0.3 aC |
12.7 ± 0.0 aAB |
15.0 ± 0.2 aA |
| Bioaccessible |
nd bλ |
46.3 ± 1.8 bλ |
28.8 ± 1.6 bλ |
24.9 ± 0.2 bλ |
| Yogurt with I. galbana freeze-dried biomass |
Initial |
59.1 ± 0.3 aC |
12.9 ± 1.1 aD |
20.2 ± 2.0 aAC |
7.8 ± 2.1 aAC |
| Bioaccessible |
nd bλ |
44.7 ± 4.5 bλ |
32.1 ± 3.2 bφλ |
23.2 ± 3.9 bλ |
| Yogurt with I. galbana ethyl acetate extract |
Initial |
52.8 ± 2.2 aAC |
16.5 ± 2.3 aD |
18.0 ± 2.2 aAD |
12.8 ± 2.2 aA |
| Bioaccessible |
nd bλ |
35.3 ± 2.0 bφ |
39.6 ± 5.8 bφ |
25.1 ± 4.9 bλ |
2.3. Fatty Acid Profile
2.3.1. Fatty Acid Profile of I. galbana Freeze-Dried Biomass and Ethyl Acetate Extract
The FA composition (in % of total FA and in mg/100 g dw) of the studied I. galbana freeze-dried biomass and ethyl acetate lipidic extract is shown in Table 3.
Table 3. Fatty acid profile (in % of total fatty acids and in mg/100 g dry weight or wet weight) of I. galbana freeze-dried biomass and I. galbana ethyl acetate extract, of control yogurt, yogurt with 2% (w/w) of I. galbana freeze-dried biomass and yogurt with 2% (w/w) of I. galbana ethyl acetate extract.
| Fatty Acid |
I. galbana Freeze-Dried Biomass |
I. galbana Ethyl Acetate Extract |
Control Yogurt |
Yogurt with I. galbana Freeze-Dried Biomass |
Yogurt with I. galbana Ethyl Acetate Extract |
| % Total Fatty Acids |
mg/100 g Dry Weight |
% Total Fatty Acids |
mg/100 g Dry Weight |
% Total Fatty Acids |
mg/100 g Wet Weight |
% Total Fatty Acids |
mg/100 g Wet Weight |
% Total Fatty Acids |
mg/100 g Wet Weight |
| 14:0 |
5.5 ± 0.4 a |
1060 ± 66 A |
13.8 ± 0.1 b |
2446 ± 17 B |
12.6 ± 0.3 b |
229 ± 6 C |
12.3 ± 0.6 cb |
268 ± 13 C |
12.5 ± 0.4 b |
255 ± 9 C |
| 16:0 |
9.3 ± 0.5 a |
1777 ± 87 A |
11.3 ± 0.2 b |
1997 ± 37 B |
34.4 ± 0.3 c |
626 ± 5 C |
32.4 ± 0.3 d |
706 ± 7 C |
33.2 ± 0.1 e |
676 ± 3 C |
| 18:0 |
0.8 ± 0.0 a |
145 ± 0 A |
0.8 ± 0.0 a |
133 ± 3 A |
9.2 ± 0.3 b |
168 ± 5 B |
8.9 ± 0.3 b |
195 ± 7 C |
9.2 ± 0.2 b |
187± 4 C |
| Σ SFA 1 |
18.8 ± 0.7 a |
3598 ± 137 A |
29.3 ± 0.5 b |
5183 ± 80 B |
63.8 ± 0.4 c |
1147 ± 3 C |
60.5 ± 0.8 d |
1319 ± 17 D |
62.1 ± 0.6 cd |
1264 ± 13 DC |
| 16:1 ω7 |
4.3 ± 0.0 a |
827 ± 7 A |
5.7 ± 0.0 b |
1004 ± 4 B |
1.8 ± 0.0 c |
33 ± 0 C |
2.2 ± 0.0 d |
48 ± 1 D |
2.0 ± 0.0 e |
41 ± 1 D |
| 18:1 ω9 |
21.0 ± 0.1 a |
4015 ± 16 A |
19.8 ± 0.5 a |
3501 ± 81 B |
19.9 ± 0.4 a |
362 ± 7 C |
21.0 ± 0.7 a |
457 ± 16 D |
20.5 ± 1.0 a |
418 ± 21 CD |
| 18:1 ω7 |
1.3 ± 0.0 a |
247 ± 3 A |
1.1 ± 0.0 ba |
189 ± 3 B |
2.0 ± 0.2 ca |
37 ± 4 C |
2.1 ± 0.1 ca |
46 ± 3 C |
1.8 ± 0.4 ca |
36 ± 9 C |
| 20:1 ω11 |
0.9 ± 0.1 a |
175 ± 9 A |
0.8 ± 0.0 a |
136 ± 1 B |
0.2 ± 0.1 b |
4.0 ± 1.7 C |
0.3 ± 0.0 b |
7.1 ± 0.5 C |
0.2 ± 0.1 b |
4.5 ± 1.9 C |
| 22:1 ω11 |
0.5 ± 0.0 a |
97 ± 3 A |
0.1 ± 0.0 b |
15 ± 0 B |
nd b |
nd B |
ndb |
nd B |
nd b |
nd B |
| Σ MUFA 2 |
28.7 ± 0.0 a |
5497± 8 A |
27.9 ± 0.4 a |
4932± 71 B |
24.0 ± 0.6 b |
401 ± 8 C |
25.7 ± 0.9 b |
560 ± 19 D |
24.7 ± 0.7 b |
502 ± 15 D |
| 16:2 ω4 |
0.3 ± 0.0 a |
57 ± 1 A |
0.4 ± 0.0 b |
65± 0 B |
0.2 ± 0.0 c |
2.9 ± 0.1 C |
0.2 ± 0.0 c |
3.4 ± 0.1 C |
0.2 ± 0.0 c |
3.4 ± 0.8 C |
| 18:2 ω6 |
12.1 ± 0.1 a |
2313 ± 14 A |
10.2 ± 0.2 b |
1806 ± 31 B |
2.2 ± 0.1 c |
40 ± 1 C |
3.0 ± 0.1 d |
65 ± 2 C |
2.6 ± 0.1 e |
54 ± 2 C |
| 18:3 ω3 |
11.8 ± 0.1 a |
2260 ± 10 A |
10.1 ± 0.1 b |
1779 ± 17 B |
0.6 ± 0.0 c |
11.5 ± 0.7 C |
1.4 ± 0.1 c |
30 ± 2 C |
0.8 ± 0.6 c |
16.9 ± 12.2 C |
| 20:4 ω3 |
0.2 ± 0.0 a |
35 ± 0 A |
0.2 ± 0.0 ab |
30 ± 0 AB |
nd b |
nd B |
nd b |
nd B |
nd b |
nd B |
| 20:4 ω6 |
0.3 ± 0.0 a |
66 ± 2 A |
0.2 ± 0.0 b |
39 ± 1 B |
0.1 ± 0.0 c |
2.0 ± 0.2 C |
0.1 ± 0.0 c |
2.8 ± 0.3 C |
0.1 ± 0.0 c |
2.7 ± 0.2 C |
| 18:4 ω3 |
10.2 ± 0.0 a |
1957 ± 3 A |
7.9 ± 0.2 b |
1387 ± 29 B |
0.6 ± 0.0 c |
10.1 ± 0.5 C |
0.8 ± 0.1 d |
16.7 ± 1.2C |
0.5 ± 0.0 c |
9.4 ± 0.8 C |
| 20:5 ω3 |
1.2 ± 0.0 a |
234 ± 1 A |
1.0 ± 0.2 a |
182 ± 28 B |
nd b |
nd C |
0.1 ± 0.0 b |
2.8 ± 0.3 C |
0.1 ± 0.0 b |
2.5 ± 0.0 C |
| 22:5 ω3 |
0.2 ± 0.0 a |
35 ± 1 A |
0.1 ± 0.0 b |
22 ± 0 B |
nd b |
nd B |
nd b |
nd B |
nd b |
nd B |
| 22:5 ω6 |
1.9 ± 0.0 a |
358 ± 5 A |
1.2 ± 0.0 b |
220 ± 7 B |
nd c |
nd C |
nd c |
nd C |
nd c |
nd C |
| 22:6 ω3 |
8.6 ± 0.2 a |
1637 ± 30 A |
5.8 ± 0.1 b |
1021 ± 18 B |
nd c |
nd C |
0.4 ± 0.1 d |
9.6 ± 1.0 C |
0.3 ± 0.0 cd |
6.2 ± 0.6 C |
| Σ PUFA 3 |
47.9 ± 0.1 a |
9154 ± 14 A |
37.8 ± 0.5 b |
6686 ± 90 B |
4.4 ± 0.1 c |
89 ± 2 C |
6.7 ± 0.2 d |
146 ± 7 C |
5.2 ± 0.4 c |
107 ± 8 C |
| Σω3 |
32.5 ± 0.1 a |
6217 ± 16 A |
25.1 ± 0.1 b |
4426 ± 15 B |
1.2 ± 0.0 c |
22 ± 1 C |
2.7 ± 0.2 d |
60 ± 4 D |
1.6 ± 0.4 c |
32 ± 9 C |
| Σω6 |
14.9 ± 0.0 a |
2847 ± 1 A |
12.2 ± 0.6 b |
2159 ± 104 B |
2.7 ± 0.1 c |
48 ± 2 C |
3.4 ± 0.1 d |
74 ± 1 C |
3.1 ± 0.1 c |
63 ± 2 C |
| Σω3/Σω6 |
2.2 ± 0.0 a |
2.2 ± 0.0 A |
2.1 ± 0.1 a |
2.1 ± 0.1 A |
0.4 ± 0.0 b |
0.4 ± 0.0 B |
0.8 ± 0.1 c |
0.8 ± 0.1 C |
0.5 ± 0.1 b |
0.5 ± 0.1 B |
The fatty acid composition (in % of total fatty acids and in mg/100 g wet weight) of the control yogurt and functional yogurts is shown in Table 3.
SFA presented the highest share and displayed values between 60 and 64% of the total FA in control yogurt and functional yogurts. The same proportions in terms of abundances (SFA > MUFA > PUFA) in microalgae fortified yogurts were also verified by other authors
[24][25]. PUFAs were the least abundant group of FAs, and ω6 PUFAs contents largely exceeded ω3 PUFAs, yielding an ω3/ω6 ratio lower than 1 in both functional yogurts. The incorporation of
I. galbana freeze-dried biomass and ethyl acetate extract induced some differences when compared to the control, namely oleic acid, linoleic acid, and ALA contents were increased. Additionally, DHA and EPA contents were detected in both functional yogurts.
2.4. Fatty Acid Bioaccessibility
The lipid bioaccessibility of
I. galbana freeze-dried biomass was 13.2 ± 1.2%, indicating a low lipid availability for absorption at the intestine. This result is in agreement with Bonfanti
et al. [10], who used the same digestion model for the same microalga. The highest bioaccessibility was detected for oleic acid (18:1 ω9), 27.5 ± 0.1%. DHA (22:6 ω3) was found to be barely bioaccessible (1.9 ± 0.0%) and EPA bioaccessibility was also low (5.7 ± 0.0%). The determined DHA and EPA bioaccessibility was lower than what Bonfanti et al.
[10] reported.
The lipid bioaccessibility of the functional yogurts was high (exceeding 86%). In the control yogurt and both functional yogurts, the palmitic acid (16:0) was highly bioaccessible (>100%). Oleic acid (18:1 ω9) was more bioaccessible in the yogurt with ethyl acetate extract (18 ± 0.9%). Among the main FA, only palmitic acid (16:0) followed the pattern of bioaccessibility enhancement after
I. galbana freeze-dried biomass and ethyl acetate extract incorporation in the yogurt matrix, which was also verified by Paulo
et al.’s
[26] study, which used the microalga
Aurantiochytrium sp. in the development of a skimmed functional yogurt. Linoleic acid (18:2 ω6) showed a low bioaccessibility in all yogurts (<10%) and stearidonic acid (18:4 ω3) bioaccessibility was higher in the functional yogurt with ethyl acetate extract (69.2 ± 6.3%). No DHA or EPA content was found to be bioaccessible in the functional yogurts, despite their initial presence.
The observed bioaccessibility loss as a result of
I. galbana incorporation is unexpected, since various studies considered yogurts an ideal food matrix for incorporating ω3 LC-PUFAs, making them more bioaccessible/bioavailable due to the preformed emulsions
[3][27][28]. In view of the low bioaccessibility of ω3 LC-PUFAs, explanations must be found.
Though the direct use of microalgal biomass in nutrition has been advised owing to supposed high assimilation levels, there seems to be a higher difficulty in humans digesting microalgal biomass. The main critical difficulty in absorption is the microalgal cell wall, which is not degraded by the digestive enzymes present in the mouth, stomach, and small intestine. Therefore, since the absorption of nutritional compounds generally occurs in the small intestine, an intact microalgal cell wall may act as a natural physical barrier for (lipophilic) nutrients, thus limiting the digestibility of intracellular nutrients as well as the bioaccessibility of health-beneficial components
[29].
The lower ω3 LC-PUFA bioaccessibility could result from a possible chemical interaction between lipids and other components from microalga. The microalgal FA released after hydrolysis may have a higher affinity for non-bioaccessible fractions components, such as polysaccharides present in the microalgae cell wall, or proteins and salts (such as calcium), establishing complexes and precipitating in the
pellet (non-digested fraction)
[8][10]. This was verified by Zhang
et al. [30], who reported that some of the FA in the microalga
Chlorella were attached to the cell wall and linked to carbohydrates by an ether bond. Therefore, since microalgal polar lipids are located in the cell membrane and in
I. galbana, the main portion of DHA was found to be present in the polar fraction; this can explain why this ω3 LC-PUFA was not bioaccessible.
Since DHA and other ω3 LC-PUFAs are highly unsaturated, this may lead to a lower bioaccessibility percentage
[10][31], which can also explain the low fatty acid bioaccessibility detected in this study.
A low lipid bioaccessibility means a lower nutritional value regarding ω3 LC-PUFAs content in the functional yogurts, but to achieve a higher level of bioaccessible LC-PUFAs, a much higher quantity of I. galbana freeze-dried biomass and ethyl acetate extract added to the yogurt than only 2% w/w would be needed. This would be unfeasible because of the impact on sensory properties. Therefore, it is important to find solutions to enhance the lipid bioaccessibility of microalgal ω3 LC-PUFAs for humans.
Some recent studies have used different techniques to increase ω3 LC-PUFAs contents and bioaccessibility. For instance, Señoráns
et al. [32] used ultrasound-assisted extraction (UAE) with different temperatures and extraction times as an alternative method to the traditional lipid extraction techniques from the microalga
I. galbana. In fact, UAE is considered a simple, economical and eco-friendly technique, thereby increasing the purity of the final product
[33][34]. The study performed by Bernaerts
et al. [29] proved the importance of cell disruption of the microalgae
Nannochloropsis sp. for the
in vitro lipid digestibility and bioaccessibility of ω3 LC-PUFAs, by using high pressure homogenization for cell disruption, which resulted in complete lipid digestibility and an increase in bioaccessibility. Prior to this, Cavonius
et al. [35] subjected the microalga
Nannochloropsis oculata to a pH-shift treatment process, which increased the accessibility of lipids after in vitro digestion. Enzyme-assisted lipid extraction techniques have also been reported
[33][34]. Therefore, the formulation of functional food products enriched with microalgae biomass needs to consider a previous microalgal cell disruption.
3. Conclusions
The obtained results show the potential of incorporating the microalga I. galbana freeze-dried biomass as a functional ingredient into yogurts, wherein ω3 LC-PUFAs content was enhanced (60 mg/100 g ww), specifically concerning DHA (9.6 mg/100 g ww), and the ω3/ω6 ratio rose to 0.8. The incorporation of I. galbana freeze-dried biomass in yogurts was shown to be more effective in enhancing ω3 LC-PUFAs content (mainly DHA) than the ethyl acetate extract incorporation, which means that the green solvent lipid extraction from I. galbana was not as effective as expected. The in vitro digestion showed a poor bioaccessibility of ω3 LC-PUFAs, with no DHA or EPA present in the bioaccessible fractions, hence indicating a low lipid bioaccessibility. Nevertheless, when compared to the original yogurt, an added-value novel functional yogurt with DHA and a higher ω3 LC-PUFAs content was obtained, which can be considered important from a nutritional point of view and a suitable source of essential FAs in the human diet.
This study was able to prove the high potential of the microalga I. galbana as a functional ingredient, showing the importance of considering bioaccessibility in the evaluation of the nutritional value of microalgae-based functional foods, since microalgal bioactive compounds were poorly bioaccessible and only a small portion of the nutrients are ready for absorption. Therefore, future work and research are required to increase I. galbana lipid digestibility and enhance ω3 LC-PUFAs’ (mainly DHA and EPA) bioaccessibility/bioavailability to humans, with microalgae cell-disruption pretreatments (such as high-pressure homogenization or ultrasound-assisted extraction) being possible solutions. Moreover, for the viability of a future functional food, a sensory acceptability study will be fundamental.