Secreted frizzled-related protein 5 (SFRP5) is an anti-inflammatory adipocytokine member of the SFRP family that is an antagonist of the WNT (wingless-MMTV integration site) family member 5a (WNT5A), a ligand of WNT pathway. There are two types of WNT signaling pathways: canonical and noncanonical, but SFRP5 is implicated only in the last one. The noncanonical pathway is activated by WNT ligands that bind to the frizzled receptors, followed by the phosphorylation of Jun N-terminal kinase (JNK) that triggers proinflammatory and proliferative processes. The SFRP5/WNT5A-mediated noncanonical pathways are associated with the pathogenesis of many inflammation-related diseases. However, SFRP5 has a controversial role in liver disease.
1. Introduction
Nonalcoholic fatty liver disease (NAFLD) is a chronic metabolic disorder of the liver that has emerged as a major public health concern with a high prevalence rate ranging from 12.9% to 46.0%, depending on the country
[1]. NAFLD represents a continuum of liver abnormalities from simple steatosis (SS) to nonalcoholic steatohepatitis (NASH) that can lead to cirrhosis and liver cancer, emerging as an important cause of liver transplant
[2][3][4]. The pathogenesis of NAFLD is complicated and involves environmental and genetic factors, hormone secretion, lipid peroxidation damage, immunological reactions, oxidative stress, abnormal fat metabolism, intestinal dysbiosis, and angiogenesis, which ultimately induce NASH and cirrhosis
[5]. However, these mechanisms are not well understood, and effective methods for preventing and treating NAFLD are still lacking
[5]. Therefore, improving the knowledge of the molecular pathways involved in NAFLD pathogenesis is crucial to identify future therapeutic targets.
Secreted frizzled-related protein 5 (SFRP5) is an anti-inflammatory adipocytokine member of the SFRP family
[6][7] that is an antagonist of the WNT (wingless-MMTV integration site) family member 5a (WNT5A), a ligand of WNT pathway
[8]. There are two types of WNT signaling pathways: canonical and noncanonical, but SFRP5 is implicated only in the last one. The noncanonical pathway is activated by WNT ligands that bind to the frizzled receptors, followed by the phosphorylation of Jun N-terminal kinase (JNK) that triggers proinflammatory and proliferative processes
[8][9].
The SFRP5/WNT5A-mediated noncanonical pathways are associated with the pathogenesis of many inflammation-related diseases
[10]. Although it has been reported that WNT signaling is activated during hepatic fibrosis, little is known about its precise mechanism in the liver
[11]. Additionally, it is also known that the phosphorylation of JNK is implicated in cell death, cancer, T2DM, and obesity and plays an important role in the activation of liver fibrosis
[12]. Therefore, it seems that SFRP5/WNT5A-mediated noncanonical may be involved in NAFLD pathogenesis (
Figure 1).
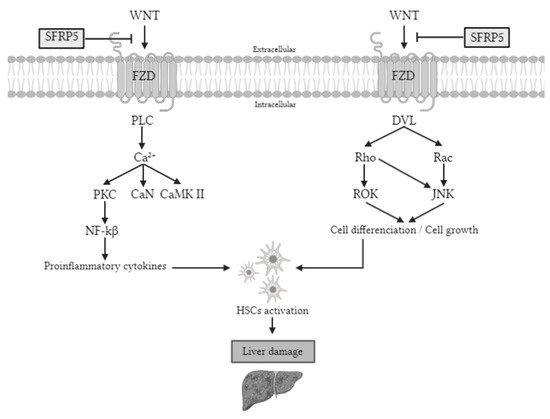
Figure 1. Noncanonical WNT pathway signaling. The WNT signaling pathway activates proteins that promote the synthesis of proinflammatory cytokines and cell differentiation. These two processes cause the activation of HSCs, which will cause liver damage if the stimulation persists. However, SFRP5 is able to inhibit the WNT signaling pathway, so the pathway is inactivated, so the liver is protected. PLC, phospholipase C; Ca2+, calcium 2+; PKC, protein kinase C; CaN, serine/threonine-protein phosphatase; CaMK II, Ca2+/calmodulin-dependent protein kinase II; NF-kβ, nuclear factor kappa-light-chain-enhancer of activated B cells; DVL, disheveled; ROK, rho-associated protein kinase; JNK, Jun N-terminal kinase; Wnt, wingless-MMTV integration site; Sfrp5, secreted frizzled-related protein 5; FZD, frizzled receptor; HSC, hepatic stellate cells.
In this sense, some studies have shown that SFRP5 can regulate metabolic disorders by improving hepatic lipid metabolism, inhibiting the growth of adipocytes
[13][14], and alleviating hepatic steatosis
[6][9]. Regarding animal models, previous studies reported that SFRP5 is a protective adipokine for glucose tolerance, adipose inflammation, hepatic steatosis, and fibrosis, and its anti-inflammatory role is perturbed in animal models of obesity with T2DM
[14][15][16]. However, the role of SFRP5 in humans is not clear.
2. Baseline Characteristics of Subjects
The clinical characteristics and biochemical measurements of the population are shown in Table 1. We first classified our cohort depending on their body mass index (BMI) as NW (BMI < 25 kg/m2, n = 20) and MO (BMI > 40 kg/m2, n = 69). Then, those with MO were subclassified according to hepatic histology as normal liver (NL; n = 28), SS (n = 24), and NASH (n = 17), which were comparable in terms of weight, BMI, insulin, homeostatic model assessment method-insulin resistance (HOMA2-IR), glycosylated hemoglobin (HbA1c), cholesterol, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), TG, aspartate aminotransferase (AST), gamma-glutamyltransferase (GGT), systolic blood pressure (SBP), and diastolic blood pressure (DBP).
Table 1. Anthropometric and biochemical variables of women in the studied cohort.
| MO (n = 69) |
| |
NW |
NL |
SS |
NASH |
| Variables |
(n = 20) |
(n = 28) |
(n = 24) |
(n = 17) |
| Weight (kg) |
54.20(51.00–64.25) |
116.50(107.25–130.50) * |
113.20(108.33–128.00) & |
112.00(104.65–125.00) ^ |
| BMI (kg/m2) |
21.97(20.47–23.90) |
43.30(40.94–46.47) * |
44.35(40.82–46.83) & |
44.46(40.76–46.03) ^ |
| Glucose (mg/dL) |
90.00(84.00–97.00) |
85.50(76.25–93.00) |
93.50(85.75–107.00) $ |
91.00(82.50–101.20) |
| Insulin (mUI/L) |
7.80(4.80–9.90) |
9.43(5.59–16.21) |
11.27(7.81–14.51) & |
6.57(5.09–23.04) |
| HOMA 2-IR |
1.05(0.60–1.30) |
1.23(0.75–2.05) |
1.49(0.95–2.18) |
0.86(0.61–3.00) |
| HbA1c (%) |
4.75(4.50–5.03) |
5.40(5.30–5.70) * |
5.60(5.25–6.03) & |
5.50(5.20–6.10) ^ |
| Cholesterol (mg/dL) |
193.98 ± 30.66 |
171.88 ± 36.20 |
174.42 ± 35.41 & |
185.28 ± 43.39 |
| HDL-C (mg/dL) |
63.75 ± 16.03 |
40.84 ± 9.89 * |
42.56 ± 12.38 & |
38.89 ± 8.47 ^ |
| LDL-C (mg/dL) |
109.99 ± 30.67 |
108.16±27.94 |
104.39 ± 31.21 |
104.62 ± 31.58 |
| TG (mg/dL) |
79.50(49.50–149.25) |
106.00(89.00–136.00) * |
129.50(85.75––175.50) & |
140.00(106.00–247.00) ^ |
| AST (UI/L) |
19.50(15.75–23.00) |
20.50(15.75–36.25) |
23.00(17.00–35.00) |
24.00(17.00–43.00) ^ |
| ALT (UI/L) |
15.00(12.00–21.00) |
22.00(16.00–27.00) * |
31.00(23.00–35.75) $,& |
30.00(15.50–40.00) ^ |
| GGT (UI/L) |
12.00(9.00–20.00) |
18.00(16.00–27.00) |
21.00(16.25–30.50) & |
25.00(15.00–27.00) ^ |
| ALP (Ul/L) |
54.44 ± 14.10 |
60.42 ± 13.09 |
75.80 ± 11.66 $,& |
62.77 ± 11.16 ” |
| SBP (mmHg) |
118.56 ± 10.92 |
119.00 ± 18.26 |
120.09 ± 13.41 |
113.44 ± 13.96 |
| DBP (mmHg) |
72.00(68.50–75.00) |
63.00(57.75–75.75) |
62.00(59.00–72.50) |
65.50(56.75–70.75) |
MO, morbid obesity; NW, normal weight; NL, normal liver; SS, simple steatosis; NASH, nonalcoholic steatohepatitis; BMI, body mass index; HOMA2-IR, homeostatic model assessment method-insulin resistance; HbA1c, glycosylated hemoglobin; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; TG, triglycerides; AST, aspartate aminotransferase; ALT, alanine aminotransferase; GGT, gamma-glutamyltransferase; ALP, alkaline phosphatase; SBP, systolic blood pressure; DBP, diastolic blood pressure. Data are expressed as the mean ± standard deviation or median (interquartile range) depends on the distribution of the variables. * Significant differences between the NW group and the NL group (p < 0.05). & Significant differences between the NW group and the SS group (p < 0.05). ^ Significant differences between the NW group and the NASH group (p < 0.05). $ Significant differences between the NL group and the SS group (p < 0.05). ” Significant differences between the SS group and the NASH group (p < 0.05).
Biochemical analyses indicated that patients with NW had significantly lower weight, BMI, HbA1c, TG, and alanine aminotransferase (ALT) than NL, SS, and NASH groups; also, levels of insulin, GGT, and alkaline phosphatase (ALP) were lower in NW compared to SS; meanwhile, AST and GGT levels of NW subjects were lower compared to NASH patients. On the other hand, our NW subjects presented higher levels of HDL-C than NAFLD patients (NL, SS, and NASH) and enhanced levels of cholesterol than those with SS. In the MO cohort, we found higher levels of glucose, ALT, and ALP in SS subjects than in the NL group and increased levels of ALP in SS than in the NASH group. It is important to note that any of the patients of our cohort who presented T2DM neither need pharmacological treatment since it has been reported that treatments for diabetes alter SFRP5 levels
[13].
3. Evaluation of Serum SFRP5 Levels According to BMI and Hepatic Histology
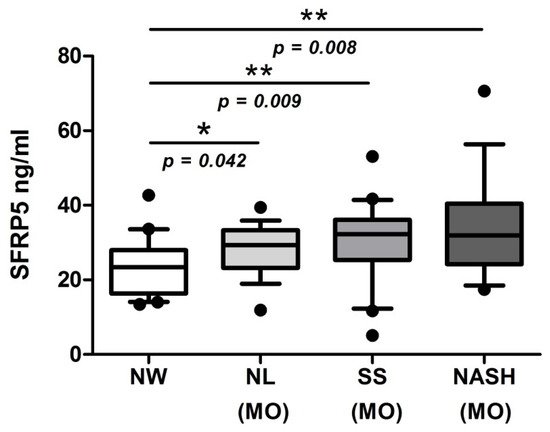
To achieve the main objective of this study, we evaluated serum SFRP5 levels in a cohort of women with NW and MO (NL, SS, and NASH). Our results showed significantly lower levels of SFRP5 in NW patients than those with MO, specifically in NL, SS, and NASH subjects, as shown in Figure 2. Unfortunately, we did not find significant differences in SFRP5 levels between NL, SS, and NASH groups.
Figure 2. Box plot graphical representation of serum levels of SFRP5 women with NW and women with MO subclassified according to NL, SS, and NASH. NW, normal weight; MO, morbidly obesity; NL, normal liver; SS, simple steatosis; NASH, nonalcoholic steatohepatitis; SFRP5, secreted frizzled-related protein 5. Individual data points outside the maximum and minimum limits represent outliers. p < 0.05 was considered statistically significant (* means p < 0.05; ** means p < 0.01).
4. Evaluation of Relative mRNA Abundance of SFRP5 in Liver According to Hepatic Histology
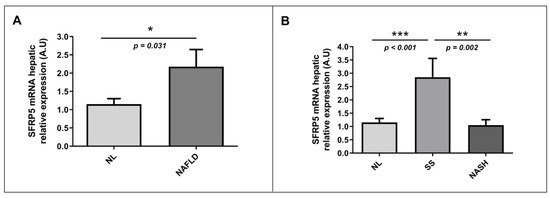
Apart from determining SFRP5 levels in our study cohort, to achieve the main objective of this study, we also evaluated the relative mRNA hepatic abundance of SFRP5 in a cohort of women with MO without or with NAFLD (SS and NASH). First, we classified our patients into NL and NAFLD, and we observed that the hepatic relative mRNA expression of SFRP5 was higher in the NAFLD group than in NL subjects (Figure 3A). Then, when we analyzed hepatic relative mRNA expression of SFRP5 according to different degrees of NAFLD, we found that SFRP5 expression was significantly higher in patients with SS than those with NL or NASH. However, hepatic relative mRNA abundance of SFRP5 did not show significant differences between NL and NASH subjects (Figure 3B).
Figure 3. Differential relative mRNA abundance of SFRP5 in liver between (A) women with MO with NL histology and women with MO with NAFLD and (B) MO women with NL, SS, and NASH. A.U, arbitrary units; MO, morbid obesity; NAFLD, nonalcoholic fatty liver disease; NL, normal liver; SS, simple steatosis; NASH, nonalcoholic steatohepatitis; SFRP5; secreted frizzled-related protein 5. p < 0.05 was considered statistically significant (* means p < 0.05; ** means p < 0.01; *** means p < 0.001).
5. Evaluation of the Relative mRNA Abundance of SFRP5 in Liver According to Liver Inflammation-Related Parameters
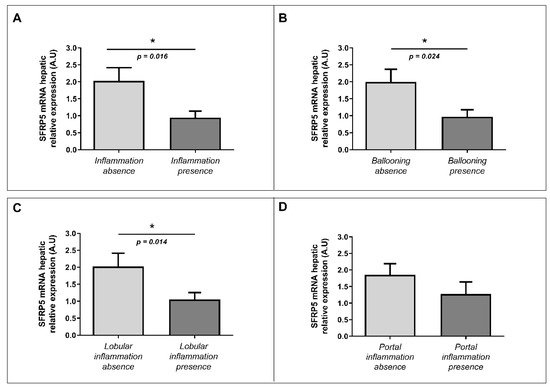
First, we wanted to explore the implication of SFRP5 in hepatic inflammation and ballooning processes analyzed under the microscope by an expert pathologist. In this regard, we observed decreased levels of mRNA abundance of SFRP5 in patients with liver inflammation than those without it (Figure 4A). We also found lower levels of mRNA relative abundance of SFRP5 in subjects with hepatic ballooning than those in the absence of it (Figure 4B). Finally, we observed low levels of hepatic mRNA SFRP5 abundance in patients with lobular inflammation than subjects without lobular inflammation (Figure 4C). There were no significant differences in SFRP5 mRNA hepatic relative expression according to portal inflammation presence or absence (Figure 4D).
Figure 4. Differential relative mRNA hepatic expression of SFRP5 between different parameters related to inflammation (ballooning, portal inflammation, and lobular inflammation). Differential relative mRNA abundance of SFRP5 in liver between (A) absence of inflammation and inflammation presence; (B) ballooning absence and ballooning presence; (C) lobular inflammation absence and presence; and (D) portal inflammation absence and presence. A.U, arbitrary units; SFRP5; secreted frizzled-related protein 5. p < 0.05 was considered statistically significant (* means p < 0.05).
6. Hepatic Expression of Validated Proinflammatory Molecules in SS and NASH Groups
Then, to corroborate the veracity of the expression peak of SFRP5 in SS samples and the downregulation in NASH ones, we have also analyzed the hepatic expression of IL-6 and TNF-α, well-known proinflammatory molecules, in SS and NASH groups. Therefore, we found significant differences in IL-6 and TNF-α expressions between SS and NASH (p = 0.033, p = 0.006, respectively), as expected.
7. Correlations of Relative Hepatic mRNA Abundance of SFRP5 with Clinical and Biochemical Parameters and with the Studied Adipocytokines
To deepen the knowledge of the role of SFRP5 in NAFLD pathogenesis, we wanted to analyze correlations between SFRP5 relative expression in the liver with different parameters such as weight, BMI, glucose, insulin, cholesterol, HDL-C, LDL-C, TG, and liver transaminases. In the correlation analysis, we only found a significant positive association between SFRP5 hepatic relative expression and ALP levels (rho = 0.404, p = 0.016).
8. Evaluation of Relative mRNA Abundance of WNT5A and JNK in Liver According to Hepatic Histology
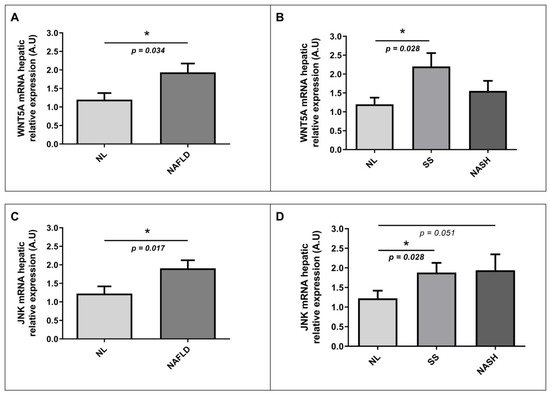
To explore the implication of the WNT signaling pathway in NAFLD pathogenesis, we also wanted to analyze in our study cohort the hepatic mRNA abundance of WNT5A and JNK, two of the main genes involved in the WNT pathway together with SFRP5. On the one hand, we observed significantly higher mRNA relative expression of WNT5A in the liver of NAFLD patients than those with NL histology, as shown in Figure 5A. Moreover, when we analyzed the hepatic relative mRNA abundance of WNT5A according to different degrees of NAFLD, we found that WNT5A hepatic expression was significantly enhanced in patients with SS than those with NL or NASH (Figure 5B). On the other hand, we observed higher levels of hepatic JNK relative expression in NAFLD patients than NL subjects (Figure 5C). Additionally, we also found increased levels of hepatic mRNA abundance of JNK in SS patients than those with NL, as was graphically represented in Figure 5D.
Figure 5. Differential relative mRNA abundance of WNT5A in liver between (A) women with MO and NL histology and women with MO with NAFLD; (B) women with MO with NL histology, women with MO with SS, and women with MO with NASH. Differential relative mRNA abundance of JNK in liver between (C) women with MO with NL histology and women with MO with NAFLD; (D) women with MO with NL histology, women with MO with SS, and women with MO with NASH. A.U, arbitrary units; MO, morbidly obesity; NAFLD, nonalcoholic fatty liver disease; NL, normal liver; SS, simple steatosis; NASH, nonalcoholic steatohepatitis; WNT5A, WNT family member 5a; JNK, Jun N-terminal kinase. p < 0.05 was considered statistically significant (* means p < 0.05).