2.1. Fruit Quality
According to Barrett et al. (2010)
[37], in reference to fruits, the characteristics that impart a distinctive quality may be described by four different attributes: color and appearance, flavor (taste and aroma), texture, and nutritional value. All these aspects are determined through the complex biological process of fruit development and ripening
[38][39].
2.2. Antioxidant Capacity
In plants, phenolic compounds are produced as secondary metabolites exerting various protective roles and are generally involved in the defense against stress conditions
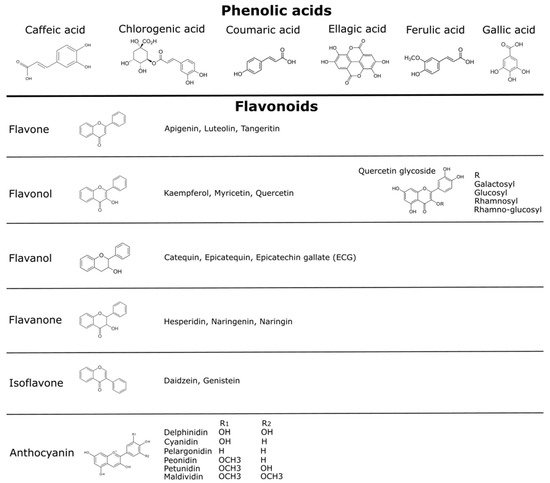
[40][41][42][43]. The main phenolic compounds in these fruits can be divided into phenolic acids, and flavonoids such as flavonols, flavanols, and anthocyanins (Figure 1)
[42][43]. These molecules are responsible for the major organoleptic characteristics of plant food, such as the visual appearance, flavor, bitterness, astringency, and aroma
[44]. Many beneficial effects attributed to phenolic compounds
[44][45][46][47] have given rise to a new interest in finding plant species with a high phenolic content and relevant biological activity. Studies on the phenolic compounds of the fruits of maqui, murta, calafate, arrayán, and Chilean strawberry highlight the high antioxidant activity they present
[15][16][17][18][19][20][21][22][23] (Table 2). In the following section, we briefly summarize the available literature on the main phenolic compounds described for the Patagonian berries analyzed in this review (Table 2).
Figure 1. Polyphenols compounds described in vegetables and fruits. Different phenolic compounds have been reported in native Chilean berries, including phenolic acid, flavonoids such as quercetins—principally quercetin glycosides—and anthocyanins
[15][16][17][18][19][20][21][22][23]. More details are presented in the text. Chemical structures credits
[48].
Table 2. Antioxidant information of Patagonian berries.
|
Species Name
|
Average Antioxidant Capacity Determined by ORAC (µmol·100 g DW−1) a
|
Average Range of Total Polyphenols Compounds Content (mg GAE g−1 DW−1) a
|
Number of Non-Anthocyanin Polyphenol Compounds Reported
|
Principal Non-Anthocyanin Polyphenol Compounds
|
Number of Anthocyanin Compound Reported
|
Principal Anthocyanin Compounds
|
|
Maqui.
|
37,174 [11][49]
|
49.7 [50]
|
13 [15]
|
Quercetin, dimethoxy-quercetin, quercetin-3-rutinoside, quercetin-3-galactoside, myricetin and its derivatives (dimethoxy-quercetin) and ellagic acid [50]
|
8 [15]
|
3-glucosides, 3,5-diglucosides, 3-sambubiosides and 3-sambubioside-5-glucosides of cyanidin and delphinidin (delphinidin 3-sambubioside-5-glucoside) [20][51]
|
|
Murta
|
43,574 [11][49]
|
9.2 [19] 34.9 [49]
|
16 [15]
|
caffeic acid-3-glucoside, quercetin-3-glucoside, quercetin, gallic acid, quercetin-3-rutinoside, quercitrin, luteolin, luteolin-3-glucoside, kaempferol, kaempferol-3-glucoside, myricetin and p-coumaric acid [52]
|
11 [15]
|
delphinidin-3-, malvidin-3- and peonidin-3-arabinoside; peonidin-3- and malvidin-3-glucoside [20][52]
|
|
Calafate
|
72,425 [11][49]
|
33.9 [49] 65.5 [19]
|
36 [15]
|
quercetin-3-rutinoside, gallic- and chlorogenic acid, caffeic and the presence of coumaric- and ferulic acid, quercetin, myricetin, and kaempferol [19]
|
30 [15]
|
delphinidin-3-glucoside, delphinidin-3-rutinoside, delphinidin-3,5-dihexoside, cyanidin-3-glucoside, petunidin-3-glucoside, petunidin-3-rutinoside, petunidin-3,5-dihexoside, malvidin-3-glucoside and malvidin-3-rutinoside [19][20]
|
|
Arrayán
|
62,500 [21]
|
27.6 [19]
|
13 [15]
|
quercetin 3-rutinoside and their derivatives, tannins and their monomers [18][21]
|
8 [15]
|
peonidin-3-galactoside, petunidin-3-arabinoside, malvidin-3-arabinoside, peonidin-3-arabinoside
delphinidin-3-arabinoside, cyanidin-3-glucoside, peonidin-3-glucoside and malvidin-3-glucoside [18][19][21]
|
|
Chilean strawberry
|
N.R.
|
N.R
|
16*20** [17]
|
ellagic acid and their pentoside- and rhamnoside derivatives. quercetin glucuronide, ellagitannin, quercetin pentoside, kaempferol glucuronide.
Catechin *, quercetin pentosid *, and quercetin hexoside *
procyanidin tetramers ** and ellagitannin ** [17]
|
4 [17]
|
cyanidin 3-O-glucoside, pelargonidin 3-O-glucoside, cyanidin-malonyl-glucoside and pelargonidin-malonyl- glucoside [17]
|
The table shows the available data concerning the antioxidant capacity determined by oxygen-radical absorbing capacity (ORAC) (µmol·100 gDW−1), total polyphenols compounds content (mg GAE gDW−1), and polyphenol compounds reported in these fruits. N.R.: not reported. (*) polyphenols compounds reported in F. chiloensis ssp. chiloensis f. chiloensis and reported in (**) Fragaria chiloensis ssp. chiloensis f. patagonica. More details are given in the text. a DW, dry weight; GAE, gallic acid equivalents.
Different methods have been used for determining the total antioxidants in different vegetables and fruit, including Patagonian berries. Currently, the oxygen-radical absorbing capacity (ORAC) is a method commonly used to compare the antioxidant capacity in different foods
[11][53]. The ORAC values (as µmol per 100 g of dry weight, DW) of maqui (37,174), calafate (72,425), murta (43,574), and arrayan (62,500) berries were reported as being higher than in commercial berries such as raspberries, blueberries (
Vaccinium corymbosum ‘Bluegold’) (27,412), and blackberries cultivated in Chile
[11][21][49]. Similar trends were reported using different methods
[20]. The Trolox equivalent (TE) antioxidant capacity (TEAC) showed that maqui (88.1) and calafate (74.5) had a higher antioxidant capacity (µmol TE per gram of fresh weight, FW) compared to murta (11.7) and blueberry (14.5) fruits
[20]. The analysis by 2,2-diphenylpicrylhydrazyl (DPPH) methods showed that the antioxidant activity (mg of crude extract per liter) was higher in maqui (399.8) than in murta (82.9)
[15]. The IC
50 range of maqui extract (0.0012 and 0.0019 g L
−1) compared to the average value (0.03 g L
−1) of commercial berries cultivated in Chile, such as blueberry (
V. corymbosum), strawberry (
F. x
ananassa), and raspberry
(Rubus idaeus), indicates that a minor concentration of maqui extract is required to inhibit DPPH radicals
[54][55]. The above information represents a fundamental background supporting the idea that the Patagonian berries have good potential as a functional food, by themselves or as food ingredients.
3. Effects of Processing on Bioactive Compounds
Many native fruits are only available in determining seasons, so it is difficult to have these fresh fruits for consumption all year or away from collection sites. In general, anthocyanins are susceptible to degradation under environmental conditions, such as oxygen, heat, and changes in pH, among others
[56]. The effectiveness, uniformity, and richness of these products are dependent upon the preservation of bioactive compounds throughout the value-added chain. Native berries exhibit high water activity and are highly perishable and susceptible to microbial deterioration, enzymatic reactions, and oxidation
[31]. The effects of drying, the microencapsulation process, and juice preparation have been evaluated in maqui and murta berries. In addition, maqui and murta leaf extracts have been evaluated as ingredients to incorporate in food or coating. It was reported that the incorporation of murta leaves extracts in tuna-fish (
Thunnus tynnus) gelatin-based edible films leads to transparent films with increased protection against UV light and antioxidant capacity
[57]. The availability of new products based on maqui and murta as functional ingredients among other Patagonian berries goes hand in hand with the study of the preservation techniques of these fruits.
4. Healthy Potential of Patagonian berries
Phenolic compounds are effective antioxidants and can display various effects, including anti-microbial, anti-inflammatory, anti-mutagenic, anti-carcinogenic, anti-allergic, anti-platelet, vasodilator, and neuroprotective effects
[45][47][58]. These properties have given rise to a new interest in finding plant species with a high phenolic content and relevant biological activity. The epidemiological evidence supporting the benefits of consuming a diet rich in foods containing polyphenols is strong
[59][60][61]. In addition to the above, the richness of certain phenolic compounds present in different foods does not guarantee their absorption by the organism, which is how the bioavailability of each of them arises as one of the properties to study to correlate the intake and the effects thereof. The bioavailability appears to differ greatly among the various phenolic compounds, and the most abundant ones in our diet are not necessarily those that have the best bioavailability profile
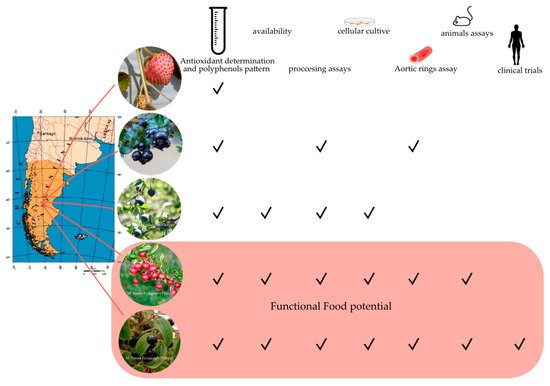
[61][62][63][64]. There has been a broad discussion about whether a high polyphenol content or high antioxidant activity can be associated with a real effect on human health. However, the results related to the preclinical evaluation of the antioxidant capacity and bioactivity of polyphenol extracts using cell cultures, isolated tissues, and animal models, before clinical trials, are still a good approach to understanding the healthy potential of several native fruits. In addition to the advances concerning characterization of the antioxidant capacity and the profile of bioactive molecules in fresh or processed Patagonian berries, advances have been made in the evaluation of the healthy potential of these berries (Figure 2). These sections summarize and discuss the literature regarding the progress in research on the effect of Patagonian fruit extracts in chronic diseases such as metabolic syndrome (MetS), diabetes, and cardiovascular diseases (CVD).
Figure 2. Summary of the Patagonian berries path to becoming functional foods. Maqui* is the native berry of Chile with major research progress concerning processing and the effect on chronic diseases. Murta* is the second most studied native berry, and two domesticated varieties are available in the market. Future studies are critical to strengthening the potential of arrayán**, calafate*, and Chilean strawberry** fruits. More details in the text. Photography credit to M. Teresa Eyzaguirre-Philippi (*) and Carlos R. Figueroa (**), map figure credit to commons.wikimedia.org/wiki/File:Pat_map.PNG, tube figure credit to
https://thenounproject.com/term/test-tube/5544/, mouse figure credit to
https://www.svgrepo.com/svg/53826/mouse, human figure credit to
https://www.flaticon.com/free-icon/standing-human-body-silhouette_30473.