1000/1000
Hot
Most Recent

Inflammatory bowel diseases (IBDs), chronic inflammatory disorders affecting the gastrointestinal tract, include Crohn’s disease and ulcerative colitis.
Inflammatory bowel diseases (IBDs), chronic inflammatory disorders affecting the gastrointestinal tract, include Crohn’s disease (CD) and ulcerative colitis (UC). Approximately 25% of IBD patients experience the onset of symptoms before 21 years of age. Pediatric IBD is considered more severe and extensive [1]. The incidence of IBD in both adults and children is increasing worldwide. According to a 2018 review, the highest incidence rates of pediatric IBD were 23 and 15.2 per 100,000 person-years in Europe and North America, respectively [2].
The etiopathogenesis of IBD results from imbalance between genetic predisposition, environmental factors (infections, diet, smoking, drugs, stress and socioeconomic status), and the gut microbiome [3]. A crucial role in bowel inflammation is played by modulation of the cytokine function. Apart from being involved in energy homeostasis, adipose tissue is an endocrine and immune organ significantly contributing to inflammatory processes. A number of adipokines (hormones and cytokines secreted by adipose tissue), especially from the mesenteric fat, can influence the pathogenesis of IBD [4]. Despite progress in this field of research, the etiopathogenetic mechanisms of IBD are not fully elucidated.
Adipose tissue is an active multifunctional metabolic organ involved in lipid storage and immunological and endocrine activity. It is composed of adipocytes, preadipocytes, macrophages, adipose-derived stromal cells, endothelial cells, fibroblasts, and leukocytes.
Adipose tissue is distributed in two main compartments, subcutaneous and visceral—see Figure 1 [5][6]. Exact differentiation of subcutaneous and visceral adipose tissue (VAT) is possible by abdominal magnetic resonance imaging or computed tomography used to measure the visceral fat area and subcutaneous fat area [7][8]. VAT could be a better predictor of disease progression and a risk factor for CD complications than obesity determined by BMI [9]. Computed tomography suggested that the ratio of the area of VAT to that of subcutaneous fat is a good marker for the CD progression and correlated with the postoperative recurrence of CD more strongly than BMI [10]. Pediatric patients with CD were observed with higher VAT volumes than healthy controls and CD-related hospitalization correlated with the increase in VAT volume [11]. Visceral adiposity, as measured by VAT volume, may be associated with a significant increase in the risk of penetrating disease and surgery in CD [12]. VAT is an independent risk factor for the endoscopic recurrence of CD after surgery. Measures of VAT may help to stratify risk in post-operative management strategies [13]. The peri-intestinal compartment of VAT, mesenteric adipose tissue, appears to play a particularly important role in IBD [6].
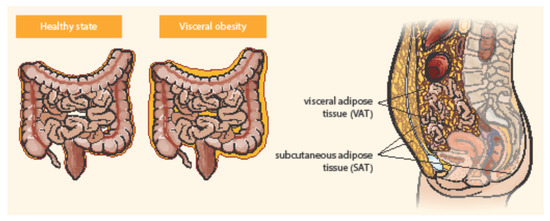
Figure 1. Distribution of abdominal adipose tissue in healthy state and visceral obesity. Adapted from Eder et al. [5].
Mesenteric adipose tissue (MAT) is a biologically very active fat compartment [6][14][15]. MAT may influence the gut barrier function by promoting the innate immune response to the gut flora. MAT may be the principal source of cytokines responsible for inflammatory processes associated with IBDs, for example interleukins (IL): IL-1β, IL-4, IL-6, IL-8, IL-10, tumor necrosis factor-α (TNF-α), adipokines, and other mediators (e.g., angiotensinogen, plasminogen activator inhibitor-1) [14][15]. The degree of cytokine expression has been shown to correlate with adipocyte mass [16]. Furthermore, preadipocytes and adipocytes can express a broad spectrum of functional innate immune receptors, namely Toll-like receptors and nucleotide-binding oligomerization domain-containing proteins 1 and 2 as a reaction to bacterial stimuli [14][17].
MAT is frequently visible by ultrasound imaging, but only magnetic resonance imaging or computed tomography (CT) enterography is an accurate and precise imaging modality for measuring both visceral and subcutaneous adipose tissue. Increased mesenteric fat density evaluated by CT enterography was found to correlate with elevated serum C-reactive protein (CRP) levels in patients with CD [18].
Bowel inflammation penetrates the surrounding adipose tissue along the mesentery in CD patients. MAT extends from the mesenteric attachment toward the root of the mesentery [3]. This hypertrophic mesenteric fat, surrounding the inflamed bowel, is called “creeping fat” or “fat wrapping”—see Figure 2 [6][14]. MAT hypertrophy was described a long time ago, in 1932, by B. B. Crohn as a consistent symptom of CD [19]. Creeping fat is pathognomonic of CD. It consisted of adipocytes, endothelial cells, immune cells, fibroblasts, pre-adipocytes, and stem cells. This activated adipose tissue secretes a broad spectrum of mediators, including cytokines, adipokines, fatty acids, and growth factors. Creeping fat is interpositioned between serosa and muscularis propria, which suggests that adipocytes are in direct contact with intestinal smooth muscle cells. In this way, the presence of creeping fat is associated with muscularis propria hyperplasia, transmural inflammation, and intestinal fibrosis. Fat wrapping, together with muscular hypertrophy and intestinal fibrosis, may participate in the stricturing form of CD [20][21].
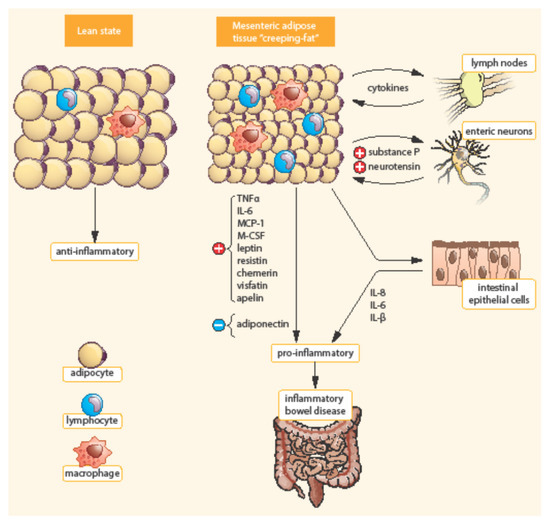
Figure 2. Differences between mesenteric adipose tissue in healthy individuals and patients with IBD. Adapted from [3]. In contrast to lean state, creeping fat is characterized by smaller, highly active adipocytes, with enhanced expression of pro-inflammatory mediators (TNF-α, IL-6, etc.). Mesenteric adipose tissue is infiltrated by immune cells (macrophages, lymphocytes) with enhanced production of chemokines (MCP-1, M-CSF). In addition, pre-adipocytes can differentiate into macrophages. TNF-α, Tumor necrosis factor α; IL, Interleukin; MCP-1, Monocyte chemotactic protein-1; M-CSF, Macrophage colony-stimulating factor.
In a retrospective study of intestinal resections, fat wrapping was found only in patients with CD and not in resections performed for other diagnoses (intestinal ischemia, tumors, etc.). Fat wrapping was positively correlated with transmural inflammation, fibrosis, stricture formation, and macrophage and lymphocyte perivascular infiltration on histology [22]. The development of mesenteric creeping fat in CD has been hypothesized to be caused by adipocyte hyperplasia rather than hypertrophy [5], resulting in an approximately four-fold increase in the number of mesenteric adipocytes compared with healthy controls [15]. There are also some observations of edematous adipose tissue and enlarged lymph nodes in patients with UC undergoing an abdomino-perineal resection [3]. However, there are probably differences in MAT from patients with ileal CD, colonic CD, and UC [23]. Creeping fat appears to be restricted to ileal specimens; it is less prominent in colonic CD and in UC. Moreover, creeping fat contains in the ileum significantly more fibrotic tissue and T cells than colonic fat from CD or UC patients. Colonic fat from CD patients shared features of both ileal fat from CD patients and colonic fat from UC patients, supporting the concept that these entities should be considered separately [5][23].
A close link and interaction between MAT and the bowel lymphatic system has been described. Lymph nodes are surrounded by perinodal adipose tissue that can also interact with lymph node cells during chronic inflammatory conditions [24]. It is supposed that impaired intestinal or mesenteric lymphatic drainage during intestinal inflammation could favor mesenteric adipocyte hyperplasia and creeping fat [3]. A recent in vitro investigation suggested that activated muscularis propria muscle cells secrete a distinct matrisome, with increased amounts of the extracellular matrix component fibronectin which, through an integrin α5ß1-mediated signaling, induces the migration of preadipocytes out of mesenteric fat and de novo formation of creeping fat [25]. Lymphatic insufficiency was demonstrated to cause adipose tissue accumulation [26]. Mesenteric lymphatic obstruction has been reported in patients with CD undergoing surgery [27].
Recently, a working model for the interactions between lymphatics, fat, and inflammation in CD has been proposed by von der Weid and Rainey [28]. Impaired lymph drainage causes increased fluid volume/pressure in the interstitium and edema. Lymph leak out of dysfunctional lymphatics induces, via lymph factors, preadipocytes to differentiate and form MAT. Conversely, both adipose deposition and interstitial fluid accumulation, alter lymphatic drainage and lead to lymphangiogenesis. Dysfunction of lymphatic vessels also impairs immune cell trafficking to lymph nodes, altering an appropriate immune response. Moreover, mesenteric adipocytes may, by their production of key chemokines in response to inflammatory/bacterial stimuli, arrange the formation of tertiary lymphoid organs rich in both T- and B- memory cells as well as plasma cells [29]. Whole transcriptional analysis also supports the role of CD-associated MAT as a site for T-, B-, and plasma cells activation, which suggests that it could act as a reservoir of memory immune response [30].
It has been shown that MAT may contribute to the development of CD by reacting to the gut microbiome. Viable bacteria were found in about 20% of mesenteric lymph nodes and adipose tissue in healthy humans, showing the physiological presence of bacteria within adipose tissue. About 95% of the total viable bacteria cultured from mesenteric tissues are physiologically located in adipocytes and only 5% are translocated to mesenteric lymph nodes, indicating that adipocytes might be the main reservoir of bacteria in the mesentery [15]. This suggests that bacteria invading MAT in the course of colitis could affect the local release of inflammatory mediators [3]. The interaction of adipocytes with gut bacteria results in adipocyte hyperplasia, induction of pro-inflammatory genes, and the secretion of chemokines attracting various leukocyte populations. The accumulation of pathogenic bacterial species in mesenteric lymph nodes drives the immune response, resulting in persistent inflammation in the MAT. This aggravates the destruction to the adjacent ileal wall, which further impairs the intestinal barrier and allows more gut bacteria to translocate to the mesentery [5].
Intestinal flora comprises bacteria, viruses, and fungi. It has complicated interactions that probably play a pathogenic role in both obesity and IBD. There are five main phyla of bacteria in the intestinal flora: Bacteroidetes, Firmicutes, Proteobacteria, Verrucomicrobi, and Actinobacteria. A shift in the microbiome composition leads to dysbiosis in visceral obesity as well as in IBD. There are some discrepancies among studies; however, major differences in bacterial phyla between obesity and IBD seem to be consistent. They comprise the increase in Firmicutes and the decrease in Actinobacteria in obese subjects compared to IBD cases [31]. In IBD, in addition to the reduction in Firmicutes, there is a quantitative and qualitative change in Bacteroidetes [32]. Apart from Actinobacteria, IBD patients have increased amounts of Enterobacteriaceae and Proteobacteria [33]. However, there exist not only differences but also similarities in the dysbiosis of obesity and IBD. In both IBD and obesity, there is an increase in Proteobacteria, Ruminococcus gnavus, and a decrease in Clostridium (leptum) and Faecalibacterium prausnitzii [31].
Compared to UC, VAT in CD is more inflamed and more colonized by intestinal commensal bacteria of type Enterococcus faecalis, increasing adipocyte proliferation [34]. In CD, as opposed to UC, the translocation of intestinal bacteria to mesenteric fat depots has been demonstrated, leading to increased CRP secretion in systemically relevant levels in these adipocytes [17]. Significant differences in the microbiota between CD and UC patients were found by using next-generation sequencing in the mesenteric lymph nodes from IBD patients undergoing bowel resection. They with CD were characterized by the overexpression of Proteobacteria (containing such pathogens as E. coli, Shigella, Salmonella, and Helicobacter spp.). Moreover, the ratio of Firmicutes-to-Bacteroides was found to be decreased in CD but increased in UC [35].
Although the pathogenic significance of dysbiosis is still not clear, changes in bacterial composition may lead to changes in gut barrier function. Due to the reduction in the number of commensal bacteria, the production of short-chain fatty acids (SCFA) is also reduced. SCFAs are a nutrient for colonocytes, which show a beneficial effect in maintaining the integrity of the intestinal barrier [36]. An important contributor is butyrate, which is a SCFA that is a product of plant polysaccharides fermentation. A low concentration of butyrate enhances the integrity of the intestinal barrier, whereas a high concentration promotes epithelial cell death [37]. The production of another SCFA, acetate, increases protection against the enteropathogen Escherichia coli 0157:H7 by maintaining epithelial barrier function and restricting the translocation of bacterial toxins to the blood supply [38]. In addition, bacterial lipopolysaccharide (LPS) may trigger low-grade inflammation that contributes to IBD, promotes an inappropriate inflammatory response—leading to an imbalance in local pro- and anti-inflammatory factors, and becomes a factor in promoting IBD [39]. These data show that probably different molecules produced by the bacterial microbiota can modulate the balance between colonic health and disease [37]. Bacteria involved in the production of SCFA such as Faecalibacterium prausnitzii, and Bifidobacteria are reduced in IBD, but reporting of such changes is somewhat variable [31].
Recently, Serena et al. tested the hypothesis that creeping fat may be a bacterial reservoir in patients with CD. They found a microbiome signature within creeping fat and MAT from CD patients, but not in subcutaneous fat. Proteobacteria was the most abundant phylum in both creeping fat and MAT, and it was positively correlated with fecal calprotectin and CRP. Notably, the clinical status of patients seemed to be related to the microbiome signature, as those with the inactive disease showed a reduction in the abundance of pathogenic bacteria. These findings demonstrated that microbiota dysbiosis associated with CD pathophysiology is reflected in VAT, which might contribute to disease by potential bacterial translocation across a disrupted intestinal barrier [40].
Adipocytes are an extrahepatic source of CRP. In a study by Anty et al., CRP gene expression was not only increased in the liver but also in the adipose tissue of obese patients compared with controls subjects. In human adipose tissue, levels of CRP mRNA were positively correlated with those of IL-6, and the CRP expression was enhanced in vitro by IL-6 and lipopolysaccharides [41]. Mesenteric fat is an important source of CRP also in CD patients. In a study by Peyrin-Biroulet et al., CRP expression was higher in the mesenteric fat of CD patients than those with UC; the samples were obtained during surgery. Increased mesenteric fat density correlated with serum CRP levels in CD [17]. CRP production by mesenteric adipocytes may be triggered by inflammatory and bacterial stimuli during bacterial translocation to mesenteric fat. It has been hypothesized that increased cytokine production in the inflamed mesentery, together with translocating bacteria, may trigger CRP production by mesenteric adipocytes in CD patients.
Moreover, the VAT expresses the whole machinery of inflammatory peptides, including classical cytokines (IL-1β, IL-6, TNF-α), chemokines (monocyte chemoattractant protein-1, C-C motif chemokine ligand 2), complement components (C1q, C3a), Toll-like receptors (TLRs), nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs), and C1q/TNF-related proteins [42]. Thus, VAT could link innate immune reactions during gut inflammation to adjacent adipose tissue alterations such as creeping fat [43]. Intestinal adipocytes residing within the VAT adjacent to inflamed gut express major functional components of the innate immune recognition system, such as TLRs and NODs/NLRs. Consequently, visceral adipocytes are able to sense a wide variety of microbial components that cross the disturbed intestinal barrier seen in IBD [44]. Thus, the observed inflammatory transformation of VAT seems to be a consequence rather than the cause of IBD. The physiological meaning behind this mechanism is most likely to provide an additional antimicrobial barrier surrounding the affected gut. This adipose tissue barrier might reduce the risk of intestinal perforation, bacterial translocation to the peritoneum, and finally, systemic inflammation and sepsis [45].
VAT also actively participates in immune responses via secretion of fat-derived hormones, so-called adipokines.