1000/1000
Hot
Most Recent

Cold atmospheric pressure plasma (CAPP) technology has received substantial attention due to its valuable properties including operational simplicity, low running cost, and environmental friendliness. Several different gases (air, nitrogen, helium, argon) and techniques (corona discharge, dielectric barrier discharge, plasma jet) can be used to generate plasma at atmospheric pressure and low temperature. Plasma treatment is routinely used in materials science to modify the surface properties (e.g., wettability, chemical composition, adhesion) of a wide range of materials (e.g., polymers, textiles, metals, glasses). Moreover, CAPP seems to be a powerful tool for the inactivation of various pathogens (e.g., bacteria, fungi, viruses) in the food industry (e.g., food and packing material decontamination, shelf life extension), agriculture (e.g., disinfection of seeds, fertilizer, water, soil) and medicine (e.g., sterilization of medical equipment, implants). Plasma medicine also holds great promise for direct therapeutic treatments in dentistry (tooth bleaching), dermatology (atopic eczema, wound healing) and oncology (melanoma, glioblastoma).
Applications of plasma technology have a long history in electronics, semiconductor industry and materials science (e.g., etching, chemical vapor deposition, plasma polymerization, surface structuring) [1][2][3][4][5]. In recent decades, cold atmospheric pressure plasma (CAPP) technology has been actively used in several industrial sectors, because it is suitable for surface treatment of many different materials (such as metal, wood, paper, glass, polymer, ceramic, nonwoven textile, etc.), while striving to retain favorable bulk properties of the material [6][7][8][9]. CAPP has recently been extended to animal and human medicine, agriculture and food industry [10][11].
Plasma is considered the fourth state of matter after solid, liquid and gas. It can be defined as a partially or fully ionized quasi-neutral substance, composed of electrons, ions, neutral particles, molecules in the ground or excited state, radical species and quanta of electromagnetic radiation (UV photons and visible light). These particles exhibit collective behavior [9]. Plasma exists in many forms in nature and constitutes more than 99% of the matter in the visible universe (stars, interstellar and interplanetary media, solar wind, tail of a comet, Aurora Borealis and Australis, quark-gluon plasma, etc.). Man-made plasma systems use heat, apply high voltage or inject electromagnetic waves to a gas for plasma generation. Plasmas are classified as equilibrium and nonequilibrium according to the relative temperature of electrons, ions and neutrals [9][12]. Equilibrium forms (e.g., torch, plasma spraying, arc jet) are known as thermal (hot) plasmas, since the temperatures of neutrals, ions and electrons are approximately of the same order maintaining a thermal equilibrium [12][13]. Nonequilibrium plasmas are known as nonthermal plasmas (cold), with the temperature of the particles varying from each other [1][14]. The electron (light particles) temperature (≈10,000 K) is much higher compared to the temperature (≈300–1000 K) of heavy species (ions and neutrals) [15][16]. Cold plasmas are weakly ionized gases, which can be generated at low as well as atmospheric pressures. They are usually excited and sustained electrically by applying radio frequency (RF) power, microwave (MW) power, alternating current (AC) and direct current (DC) [12][17][18].
Nowadays, cold atmospheric pressure plasma is considered more advantageous over low-pressure (<100 Pa) plasma for industrial applications, due to its technological and economic advantages [19]. First of all, CAPP is able to generate stable plasma at atmospheric pressure, i.e., there is no vacuum and the reactor is frequently open [6]. The application thus requires lower investment and operational cost compared to low-pressure plasma systems due to the absence of costly time-, space- and energy-consuming vacuum systems [20]. Moreover, the simplest CAPP reactors use only ambient air as working gas, which is converted into plasma (i.e., gas supply is not required) [6]. Thus, CAPP systems are easy to handle with excellent scalability and industrial applicability (integrable in existing process lines, capable of continuous surface modification, etc.) [11]. Another advantage of cold plasma is the ability to treat thermally labile samples (soft and organic materials, polymers) without any surface damage because the substrate temperature remains close to room temperature (in general < 50 °C) [15][21]. The treatment time is relatively short (from seconds to minutes) [9][15]. The efficiency of CAPP treatment depends on the reactor configuration (electrode arrangement, distance from the substrate surface) and the operating parameters of the device (gas composition, flow rate, power, temperature, process duration, etc.) [22][23][24].
There are several methods for generating CAPP from various gases such as (i) dielectric barrier discharge, (ii) plasma jet, (iii) corona discharge, (iv) gliding arc discharge, etc. (Figure 1) [10][25]. Commonly used working gas includes air, oxygen, nitrogen, helium, argon and their mixtures [14][18][21][26].
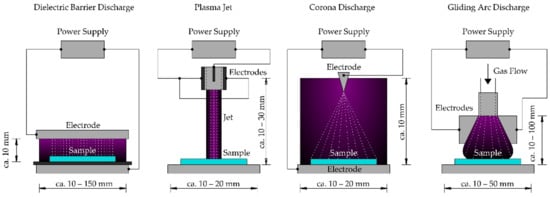
Figure 1. Schematic drawing of diverse cold atmospheric pressure plasma devices.
Dielectric barrier discharge (DBD) is generated by applying a high voltage (~kV) electric DC or AC current at high frequency (~kHz) across an adjustable gap (ranging from tens of microns to several cm) between two electrodes separated by an insulating dielectric barrier. The geometry of DBDs usually consists of two parallel plates in planar or cylindrical arrangements. DBDs use a dielectric material (e.g., quartz, glass, ceramics, enamel, silicon rubber, teflon, mica, plastic) to cover at least one of the electrodes [27]. The nonconducting coating eliminates the transition of the discharge to an electrical arc [10][28][29][30].
The atmospheric pressure plasma jet (APPJ) is a type of cold plasma discharge that produces a high velocity stream of highly reactive chemical species with weak emitted light [31][32]. APPJs typically consist of two concentric cylindrical electrodes, where the inner electrode is connected to a RF or MW power source at high frequency, causing ionization of the working gas (mainly noble gases such as helium or argon). The gas exits through a nozzle, which gives a “jet-like” appearance [20]. Based on the configuration and used materials, APPJs can be divided into single electrode jets, dielectric-free electrode jets, DBD jets, etc. Miniature plasma jets are known as plasma pens, plasma torches or plasma needles [28][30][33]. The main component of plasma needles is an electrode with a sharpened tip inside of a tube. The feed gas (most frequently helium) is flowing through a tube and is mixed with air at the needle tip where a micro discharge is created [34]. The diameter of the generated plasma glow is a few millimeters [35].
Corona discharge is generated by the application of high voltage between two or more sharp electrodes. The coronizing electrode is usually realized as a needle or a thin wire. The ionization process creates a crown around this active electrode. Coronas are very weak discharges, having very low electron and ion densities [36][37].
Gliding arc discharge plasma reactors are known as hot plasma sources, however, under specific conditions they may also produce cold plasma. The gliding arc plasma can combine the advantages of both thermal and nonthermal plasmas (nonthermal plasma conditions at higher power). The discharge is formed by a high voltage at the spot where the distance between diverging electrodes is the shortest (~ in the range of millimeters). Electrodes are placed in a fast gas flow and the discharge increases its volume and length in the flow direction [38][39][40].
The plasma sources can be applied directly (the target is in direct contact with the active plasma region) or indirectly (plasma afterglow or storable plasma-activated medium is used which contains various reactive species) to the object [41].
In the past 20 years, cold plasma treatment has been extended to medicine, mainly as a tool for the inactivation of pathogens (bacteria, fungi, viruses, biofilms) on medical or laboratory equipment (e.g., surgical instruments, pharmaceutical devices, implants, dialysis tubes, glassware, plastic tubes, pipette tips, beds, floors, etc.) [42][43][44]. Conventional methods for disinfection (inactivation of pathogenic organisms) and sterilization (elimination of all viable microorganisms), such as steam and heat treatment, autoclaves, irradiation, wet chemical treatment (ethylene oxide, ozone, chlorine, hydrogen peroxide, sodium hypochlorite, alcohol or any carbohydrate solutions, superoxidized water, various kind of acids, etc.) and UV light exhibit several drawbacks [45]. They are often slow-acting, flammable or unstable at ambient conditions [46]. Moreover, they can cause irreversible damage to the modified material and irritate the skin and eyes of humans [47]. On the contrary, CAPP can be utilized for heat labile or chemically reactive materials (e.g., heat sensitive biomaterials, polymers, living tissues) to prevent secondary infections.
Two basic types of CAPP systems are dominating in plasma medicine: indirect (e.g., plasma jet, pen, needle) and direct (e.g., dielectric barrier discharge device) plasma sources [26][36][48][49]. In the direct exposure mode, the plasma is in contact with the biological target. Floating electrode DBD (FE-DBD) system allows safe usage of plasma in therapeutic applications. It consists of two electrodes, the first is a dielectric-protected powered electrode and the second electrode is a human or animal body (skin or organ) [41][50]. Direct application of CAPP requires high standards of safety (e.g., homogeneous discharge with permitted values has to be created in order to provide nondestructive treatment). Indirect plasma sources do not use the human tissue as a counter electrode, the afterglow of plasma or plasma-activated liquid (it can be stored and shipped) is used [49][51]. Typically, these devices are portable, and allow efficient treatment of large areas even with uneven surfaces. However, their homogeneity and stability need further improvement [47].
Plasma sources produce reactive species (with a lifetime ranging from nanoseconds to hours), which are known from redox biology and play a pivotal role in biological applications [52][53][54][55][56][57]. Reactive oxygen-based species (ROS) include, e.g., superoxide (O∙−2), hydrogen peroxide (H2O2), hydroxyl radical (•OH), singlet oxygen (1O2), ozone (O3), alkoxyl (RO•) and peroxyl (RO2•). Nitrogen-based species include (RNS), e.g., nitric oxide (•NO), nitrogen dioxide (•NO2) and peroxynitrite (ONOO−). These reactive species (ROS/RNS=RONS) also play a crucial role in the pathogen inactivation mechanism (Figure 2), because they can modulate the environment of cells and affect their behavior [30][48][49][58][59]. RONS can cause cell wall erosion, cell membrane disruption (protein denaturation, virus leakage), functionality changes (oxidation of amino acids), damage to DNA and RNA and apoptosis [43][57][60]. However, it should be pointed out that the precise mechanism of microbial inactivation using CAPP treatment and the contribution of various components (UV, electrons, ion bombardment and reactive species) has not yet been fully understood [61].
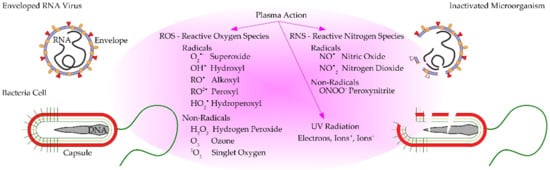
Figure 2. Schematic illustration of plasma inactivation of viruses and bacteria using CAPP treatment.
Drug resistance and incurable bacterial infections are serious public health threats, as the world is heading towards a post-antibiotic era, mainly caused by the misuse and overuse of various medications [62]. It is an urgent need to develop new techniques to treat infectious diseases caused by multidrug-resistant germs. Promising methods include light-based therapies (antimicrobial blue light, antimicrobial photodynamic inactivation, ultraviolet light, pulsed light) and CAPP [63]. CAPP in particular seems to be highly effective against multidrug resistant organisms (e.g., Methicillin-resistant Staphylococcus aureus, vancomycin-resistant enterococci), regardless of the kind of germ [42][47][49][64]. Clearly, the efficiency of the elimination process is highly dependent on the plasma device, process parameters and environmental factors (e.g., wound type, extracellular matrix) [65]. CAPP did not show any bacterial adaptation after consecutive treatments [63].
Brun et al. studied two clinically significant ESKAPE (an acronym for six virulent and antibiotic resistant pathogens) bacteria. After the plasma treatment, the decrease in bacterial load (P. aeruginosa and methicillin-resistant S. aureus) was comparable to the inactivation using biocide (chlorhexidine) and antibiotics (ciprofloxacin, daptomycin). The CAPP device (RF source from two parallel brass grids operating with helium) generated RONS in bacteria, disrupted membrane integrity and reduced the bacterial load. They treated the samples using the plasma afterglow, which inactivated the bacteria independently of their growth mode (planktonic or biofilm) [66].
Safety and bactericidal efficiency of CAPP against Pseudomonas aeruginosa was studied by Dijksteel et al. Their DBD plasma treatment (4–6 min) had bactericidal properties, but it did not induce mutations, apoptosis and DNA damage or affected the wound healing process in in vitro and ex vivo rat wound models [65].
The in vitro study of Wang et al. evaluated the bactericidal efficacy of CAPP (plasma jet using air) on a strain of super multidrug-resistant P. aeruginosa (aerobic Gram-negative bacillus causing various infections, such as pneumonia, bloodstream, skin and soft tissue infections). Both direct and plasma activated liquid treatments resulted in a decrease in cell growth (based on the colony-forming unit assay). The direct CAPP exposure was more effective, inducing higher intracellular ROS levels. Furthermore, the disruption of membrane integrity was also more effective based on immunofluorescence images [67].
Gas plasma technology has been under research for more than 50 years due to its decontaminating properties and the majority of work has been focused on bacteria. However, contamination with infectious viruses also threatens human and animal health. The antiviral activity of CAPP is a younger but evolving research area. For example, Bunz et al. observed that CAPP has an antiviral effect against herpes simplex virus type 1 (HSV-1). The effect was measurable after reducing the viral load by 100-fold [68]. Guo et al. efficiently inactivated different kinds of bacteriophages (including double-stranded DNA, single-stranded DNA and RNA bacteriophages) using CAPP treatment. Damaging of nucleic acids and proteins was achieved using argon and artificial air. Antiviral ability of plasma-activated water could also be a promising disinfection method to prevent the spread of viral diseases [56]. The paper of Filipić et al. provided a comprehensive overview about the achievements in the plasma-mediated inactivation of viruses. Their review discusses the inactivation of enteric viruses (e.g., norovirus, adenovirus, hepatitis A virus), respiratory viruses (influenza A and B, SARS-CoV-2), sexually transmitted viruses (e.g., HIV) and animal viruses (e.g., avian influenza virus, porcine reproductive and respiratory syndrome virus) [24].
During viral pandemics, like the COVID-19 crisis, respiratory viruses are responsible for over a million deaths [69]. In 2020, there has been an enormous research effort to use CAPP technology as an antiviral treatment [24]. Guo et al. used pseudoviruses with the SARS-CoV-2 S protein as a model (for biosafety limitations), and showed that plasma-activated water effectively inhibited pseudovirus infection through S protein inactivation [70]. Decontamination procedures of pathogenic viruses in different media and on various surfaces could help to limit virus spread [43]. Chen et al. studied SARS-CoV-2 inactivation using APPJ with argon and helium feed gas on various surfaces including plastic, metal and leather (the discharge voltages for argon and helium were 16.8 kV and 16.6 kV at 12.9 kHz and 12.7 kHz frequency, flow rate 6.4 L/min and 16.5 L/min). The roughness, material composition and absorptivity were aspects that influenced surface inactivation of SARS-CoV-2. The argon plasma treatment inactivated all viruses in less than 180 s. Helium plasma did not disinfect all viruses on surfaces even at 300 s because of the much lower RONS concentrations compared to argon plasma for the same operating conditions. The results confirmed the important role played by RONS concentration in virus inactivation. Comprehension of how SARS-CoV-2 interacts with CAPP is essential for future plasma utilization. [71]. The spread of SARS-CoV-2 is mostly via the transmission of respiratory droplets in poorly ventilated closed spaces [72]. It is important to try to improve the indoor air quality, e.g., by reducing the airborne time of SARS-CoV-2 aerosol microdroplets. Bisag et al. used a lab-scale dielectric barrier discharge plasma source to inactivate aerosols containing purified SARS-CoV-2 RNA suspension flowing through the device. Results show that CAP can degrade viral RNA in a short residence time (<0.2 s) [73]. The virus contains nucleic acids encased in a capsid protein coat. Reactive oxygen and nitrogen species have been shown to be responsible for virus inactivation through effects on capsid proteins, which preceded the degradation of nucleic acids. RONS can disrupt virus integrity by etching away the protein protective layer, and then act directly on nucleic acids, damaging the genetic material used for virus genome translation and replication. CAPP treatment can result in loss of viral infectivity [24][74].
Application of cold plasma is expected to decontaminate face masks or shields from SARS-CoV-2 surrogates without affecting their filtration and fitting performance [24][75]. Furthermore, CAPP can be used as an alternative to alcohol-based hand disinfection [76]. Hands are one of the most common transmission routes for many infections because people frequently touch their facial mucous membranes—the eyes, nose and mouth, which are the main portal of entry for germs [24][43]. Sterilization using CAPP is a fast and efficient method, which is essential in healthcare institutions. However, some undesirable effects have to be solved (e.g., ozone is a toxic byproduct) by optimization of the geometry of the hand dispensary system [76]. Based on early and incomplete results, CAPP has potential in inactivating different types of viruses in the near future, but confirmation will be required, because studies are in their infancy at present. Scientists anticipate that CAPP will be an effective and environmentally friendly tool to replace, complement, or upgrade existing sterilization methods, mitigating the economic and public health burdens of the pandemic [43].
Attri et al. analyzed in full theoretical detail the influence of oxidation on the SARS-CoV-2CTD protein structure and the SARS-CoV2-CTD/hACE complex. Via molecular dynamics, the basic statistical characteristics, relative binding free energy of the oxidized complex and the effect of CAPP-induced oxidation on the stability/flexibility have been determined. It was shown that the protein structures are sensitive to radical oxygen and the binding free energy (without entropic term) is lowered as a consequence of the plasma oxidation. However, the inactivation mechanism of SARS-CoV by CAPP is still an open problem, the application of cold plasma seems to be a very prospective method in this field [77].
Besides decontamination, cold plasma technology can also be used for direct therapeutic intervention. With the help of plasma, the medical treatment process could be markedly shortened. Other benefits of CAPP treatment are good tolerability (painless), biocompatibility and biosecurity (it is assumed that CAPP treatment can be applied to living cells and tissues without causing any damage) [47][78].
First of all, CAPP is a promising tool for wound healing because it has antimicrobial effects. Furthermore, it can enhance the proliferation of skin fibroblasts and angiogenesis [49][79]. Wiegand et al. demonstrated that plasma treatment using an open jet plasma device has strong bactericidal and fungicidal properties. Different bacteria strains (Staphylococcus aureus, Pseudomonas aeruginosa) and yeasts (Candida albicans and Malassezia pachydermatis) were treated which are associated with wound and skin infections. After the CAPP treatment, differences were observed due to the diverse structures of the cell wall of the microbes (e.g., Gram-positive bacteria have thicker cell walls then Gram-negative bacteria). The study concentrated on the influence of various process parameters of the plasma treatment. Nitrogen-based plasma was more effective than air. Increased input power and treatment time had higher antimicrobial efficacy against the tested microorganisms. [42].
The CAPP application has been intensively studied in dermatology [47]. Clinical trials have focused on onychomycosis (fungal infection of the nail), oncology (melanoma, actinic keratosis), viral warts (caused by infection with human papillomavirus), wound healing (different kinds of chronic infectious, microbiologically infected/contaminated wounds, herpes zoster-related pain), hair loss (increased hair follicle diameter), biofilm disruption (atopic dermatitis, itch), cosmetic applications (skin rejuvenation, reduction of wrinkles) and other ailments [26][51]. The treatment requires careful dosage (fast treatment and gentle heating) in order to prevent unwanted skin burns or damage deeper tissues. For example, longer exposure of the skin (>43 °C) can cause blisters [47].
CAPP also exhibits a promising future in dentistry for bonding to dentin and ceramics, surface activation of dental implants, tooth bleaching, removing dental plaque/biofilms from teeth, composite restoration and disinfection of root canals [80]. Plasma can reach inaccessible areas of the tooth surface such as fissures and grooves, furthermore the procedure is painless because plasma does not cause thermal damage to the tissue [41][81]. The promising effects of CAPP treatment useful in various areas of dentistry, for instance, cariology (cariogenic microorganisms, e.g., Streptococcus mutans, Lactobacillus acidophilus), periodontology (Porphyromonas gingivalis, Tannerella forsythia and Treponema denticola), endodontics (Enterococcus faecalis,) and oral oncology, was reviewed by Borges et al. [82]. Lee et al. investigated the antibacterial effect of CAPP treated dental titanium implants on Gram-positive and Gram-negative bacteria with different cell wall structures. The biofilm formation rate and adhesion of Gram-negative bacteria were significantly lower than Gram-positive bacteria on samples treated with the APPJ. Gram-positive bacteria are more resistant to plasma than Gram-negative bacteria. This is related to the thickness of the peptidoglycan layer in the bacterial cell wall, which may reduce the antibacterial effects [83].
Cold plasma has shown promising results in cancer therapy because it can selectively induce apoptosis in cancer cells, reduce tumor volume and vasculature and halt metastasis [49][58][84]. CAPP has shown substantial anticancer capability over a wide range of cancer cells, for instance, melanoma [47][85], carcinoma [86][87] and osteosarcoma [88]. Cancer cells vary from normal cells, e.g., they produce higher amounts of reactive oxygen species. Thus, cancer cells are more susceptible to plasma-generated ROS than normal cells. ROSs contribute to oxidative stress and can cause cell death [89]. However, it is important to tailor the plasma process parameters to produce a controllable amount of ROS to obtain the desired biological responses. The mechanism of CAPP action in the case of cancer includes the induction of apoptosis, DNA damage, cell cycle arrest at the S-phase and increase of the intracellular ROS concentrations [79]. CAPP can open new horizons in oncology because it seems to be a promising complementary therapy to conventional cancer treatments (chemotherapy, surgery, radiation therapy, immunotherapy and others).
The anti-melanoma activity of a softjet CAPP device was studied by Yadav et al. Three different human melanoma cells (skin cancer) were successfully treated using air and N2 gases. The results showed preferential killing of melanoma cells, apoptotic pathways by triggering apoptotic genes in all three melanoma cell lines. However, the exact mechanism for the inhibitory effect of CAPP has remained unknown [85].
Mateu-Sanz et al. provided evidence of plasma-activated Ringer’s saline (PAR) as a promising therapy option for treating Osteosarcoma (a type of malignant bone cancer). They used two types of plasma jet devices (single-electrode needle device operating in helium and pin-type electrode with a grounded outer electrode operating with argon) to obtain PAR. The cytotoxic effects of PAR in human and mouse osteosarcoma was investigated [88].
Based on the above-mentioned properties, it can be said that plasma medicine holds enormous application and development potential (Figure 3) [47]. CAPP seems to be ready to enter the healthcare area for various medical therapies. However, a lot remains to be done (conduction and evaluation of preclinical and clinical trials with standardized protocols), in order to fully understand the interactions between plasma and biological cells and tissues (both in vitro and in vivo) [26][49].
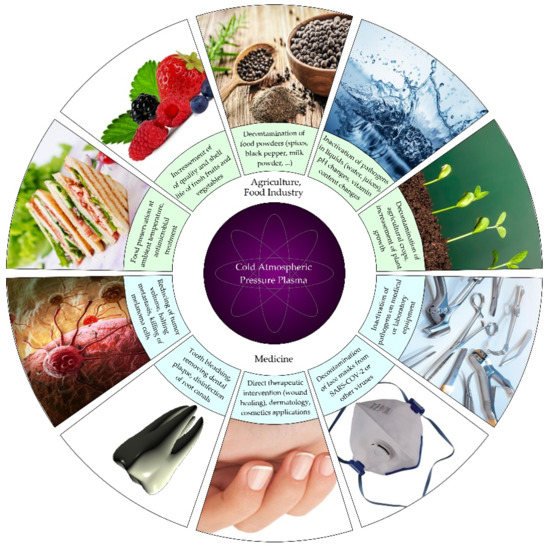
Figure 3. Schematic illustration of selected applications of CAPP treatment in medicine, agriculture and food industry.
Global food demand is significantly increasing due to the rapidly growing world population, changes in diet, limited agricultural land, climate change and increasing water deficiency [22]. Agriculture and the food industry face intense pressure because modern-day consumers desire nutritious, fresh, safe and minimally processed foods and drinks. Nowadays, a plethora of methods is available for food processing, such as pasteurization, high-pressure processing, distillation, ozonation, irradiation (ultraviolet light treatment, pulsed light treatment), ultrasonication and chemical treatments (e.g., chlorine, hydrogen peroxide, peroxyacetic acid, organic acid) [25][61][90]. However, these kinds of treatments could have a negative impact on the food quality, toxicology, sensorial and nutritive characteristics which make them less attractive to customers [25][91]. For these reasons, the food industry is continuously seeking alternative methods to improve food production [11][92].
Inactivation of microorganisms (disinfection of foods, packaging materials and equipment) improves food safety, which is one of the major challenges of this industrial sector (Figure 3) [22]. The major components of food matrices are protein, lipids, carbohydrates, water, minerals and vitamins [10]. The aim of any processing is to impart edibility, palatability and prolong shelf life, while maintaining food quality.
CAPP treatment seems to be an effective technology that achieves food preservation at ambient or sublethal temperatures, which reduces the negative thermal effects on the nutritional and quality properties of food. Cold plasma has great potential to inactivate bacteria, viruses, mycotoxins, yeast, molds, endotoxins, etc., on plant- and animal-based foods (e.g., fresh and dry fruits, vegetables, juices, nuts, egg shells, egg-based products, undercooked meat and fish, dairy products, spices, cereals) [20][23][52][90][91][93][94][95][96]. Different types of Escherichia coli, Listeria, Salmonella, Tulane virus and hepatitis A virus are the most prevalent foodborne pathogens, which can cause serious health issues [61][94][97][98][99]. Several pathogens can also survive freezing (E. coli for >180 days) [10].
The specific mechanism of microbial inactivation is still under investigation. The inactivation occurs due to various physicochemical processes (emission of UV radiation, generation of ozone and other active species) [25]. The production of reactive species depends on the used working gas. Reactive oxygen and/or nitrogen species have a potent microbicidal effect, they can damage macromolecules by oxidizing nucleic acids, proteins and lipids [24][25][93]. Besides that, the UV radiation can destroy the membranes of microorganisms, structural cell functions and genetic material of pathogens [93]. However, it is worth mentioning that the contribution of UV radiation to the antimicrobial effect is controversial [100]. The resistance to UV light seems to be dependent on the type of microorganism (microbial growth phase, stress conditions, e.g., pH, osmotic concentration and growth temperature) and the plasma device [25]. The most often used reactor configurations are DBD and APPJ. The physicochemical properties (color, texture, pH, acidity, antioxidant activity, amount of proteins, enzymes, carbohydrates, vitamins, lipids, allergens, toxins) could change depending on the plasma treatment process parameters [10][20][22]. Furthermore, the operating cost of plasma processing is an important consideration. It mainly depends on the cost of the working gas (plasma generated from noble gases is much more expensive than air) [23].
The antimicrobial effect of the plasma treatment is also highly dependent on the surface structure and surface to volume ratio of the product [23][25][57]. CAPP treatment on solid/dry foods mainly affects only their surface. The low penetration depth of the plasma components is advantageous because more important nutrients can remain inside the food [10].
Spoilage of ready-to-eat salads (fruits and vegetables) due to microbial growth causes significant food waste. The effect of DBD plasma treatment on the quality (microbial load, hardness, pH value, color) and shelf life of fresh cut, leafy rocket salad was studied by Giannoglou et al. The greatest reduction of the load (Pseudomonas spp.) was obtained after 10 min plasma treatment. The pH and color of the leaves were not affected, the shelf life was increased compared to untreated salad [101].
Yadav et al. used DBD plasma treatment for ready-to-eat ham to inactivate Listeria monocytogenes, which may occur during slicing and packing. They significantly reduced the cell counts (at least by 2 log) and studied the changes in color and lipid oxidation as a function of ham formulation, plasma treatment time, in-package gas composition and storage conditions [102].
Decontamination of food powders (e.g., onion powder, spices, black pepper, legume flour, milk powder) using plasma is more complex. Usually, it requires high plasma power density, long treatment time compared to flat samples. Pina-Perez et al. published the inactivation efficiency of Bacillus subtilis spores using an air surface microdischarge CAPP with low plasma power density (5 mW/cm2) and relatively short treatment time (7 min). The inhibiting effects on flat glass and corn starch samples were evaluated. Etching of spore hulls using reactive nitrogen species has been reported as a fundamental inactivation principle [103].
In the case of liquids, the penetration depth is less important, because every element comes into contact with the plasma volume, however, not only the pathogens are damaged [10]. Several studies confirmed that CAPP treatment has the ability to inactivate microorganisms (e.g., Escherichia coli O157:H7, Zygosaccharomyces rouxii, Salmonella enterica) in fruit juices (e.g., apple, orange, blueberry) with very good inactivation rate. Although there is some uncertainty in the literature about the operating parameters and their effect on the quality parameters of the juices. Some researchers indicated that increasing treatment time can lead to color changes (ascorbic acid degradation), pH changes and changes in vitamin content (due to oxidation reactions). Moreover, additional research is needed for large scale processing and to prove that plasma does not have any toxic residuals [91].
Many different microorganisms attach to surfaces (e.g., food matrixes, processing equipment) and develop biofilms [57]. These complex microbial ecosystems are more resistant to various environmental stresses, antimicrobials and inactivation treatments (longer treatment time is necessary) than planktonic cells due to their three-dimensional extracellular matrix [25]. Biofilm inactivation process includes various processes, like destruction of extracellular matrix, cells and cell components, etching and reduction of biofilm thickness [57]. Kadri et al. also demonstrated that biofilms are less susceptible to cold plasma treatment. Furthermore, treatment efficiency depends on the biofilm age (mature biofilms are more resistant to CAPP than young biofilms) and the type of bacteria. They presented the inactivation of various single and mixed biofilm systems of L. innocua and E. coli, which produced an extracellular polymeric substance matrix of different thickness and composition [92].
XU et al. extensively studied yeast cell inactivation at the subcellular level. It was shown that cold plasma may effectively lower the yeast cell physiological activities by the superposition of several mechanisms with different impacts (in particular, cell membrane damage, energy metabolism or DNA fragmentation). Their results showed that •OH (it attacked the cell membrane and increased its permeability) and 1O2 species (it disturbed the cell energy metabolism) contribute most to the yeast inactivation [59].
Attri et al. showed that RONS produced using CAPP is able to inactivate thermophilic bacteria, which can tolerate a wide temperature and pH range (e.g., spores of Geobacillus spp. in raw milk can survive pasteurization temperatures, spores of Bacillus stearothermophilus can spoil low acid canned foods). Sufficiently high dose (long treatment duration, i.e., 20 min) plasma treatment (using DBD device) caused protein denaturation/modification (model protein: MTH1880 from Methanobacterium thermoautotrophicum) [104].
Beyrer et al. studied the effectiveness of a direct contact DBD device against spores (dormant forms of microorganisms, like bacterial endospores), which are very resistant to heat and UV treatments due to their durable coat layers. They compared the inactivation kinetics of spores of Bacillus spp. (~3 log10 cycles of inactivation after 10 s exposure time), Geobacillus spp. and Penicillium spp. on flat glass carriers, native starch granules (non-porous material) and shells of diatoms (highly porous system) [105].
The safety of animal origin foods is an even bigger challenge for the food industry. Microbial inactivation experiments mainly focus on poultry (e.g., Salmonella spp. on eggs, chicken meat), meat (e.g., L. monocytogenes, E. coli, Salmonella spp. and C. jejuni on beef, pork) and fish (e.g., Lactobacillus, Pseudomonas) products [25]. CAPP treatment is able to inactivate various pathogens, however, ROS species can cause quality problems, which affects the consumer acceptability and shelf life [93]. For example, changes in color (e.g., loss of color, darkening) are a result of undesirable reactions due to the partial inactivation of enzymes and microorganisms [25][52]. The review of Nasiru et al. summarizes the influence of various DBD plasma device process parameters on microbial inactivation in meat products. They concluded that the use of oxygen or carbon dioxide in the working gas mixture results in enhanced effectiveness of the plasma treatment. Furthermore, increased power/voltage and treatment duration also have a pronounced effect on microbial decontamination [106]. Seafood is considered part of a healthy diet, however these products are responsible for many foodborne disease outbreaks. Seafood is shipped worldwide; the control of pathogenic and spoilage microorganisms is essential to ensure food safety. CAPP has high potential for commercial use in the seafood industry. Ekonomou et al. published a systematic review about the microbiological safety and quality of fish and seafood (squid shreds, mackerel, Asian sea bass). They compared various non thermal methods (high hydrostatic pressure processing, ultrasound, pulsed electric field, electrolyzed water and CAPP treatment) [107].
Plasma treatment is also gaining attention in agriculture since climate change has caused reductions in crop yield (reduction in the quality and quantity of crop products) (Figure 3) [108]. Furthermore, chemical-based methods are becoming less preferred due to the emergence of pathogen resistance (many pests have developed resistance to pesticides, new diseases have emerged) and environmental pollution [98]. CAP has the potential to increase crop plant vitality and production.
Agricultural crops (fruits, vegetables) are often contaminated because they come in contact with dust, insects, animal urine and feces, workers and equipment during harvest and postharvest (transport, storage, cleaning, packaging and food processing) stages [16][108]. Plasma inactivation processes are mostly focusing on bacterial (e.g., Erwinia carotovora, Clavibacter michiganensis, Pectobacterium carotovorum) and fungal pathogens (e.g., Alternaria, Aspergillus, Botrytis, Colletotrichum, Fusarium, Penicillium) in agriculture [109].
The review papers of Attri et al. focused on preharvest applications of CAPP in agriculture. These papers summarize laboratory experiments dealing with the effects of direct (DBD, APPJ) and indirect (plasma-activated water) treatments on the plant (e.g., wheat, corn, chili pepper, lentils, tomato, rice, etc.) growth and development. The CAPP generated RONS can alter the germination rate, plant morphology (shoot and root length, leaf area, etc.), gene expression and biochemical processes (changes in hormones, amino acids, antioxidants, soluble sugar level, chlorophyll content, etc.). For example, higher antioxidant activity, growth hormones and metabolites led to early germination, improved germination percentage, elevated growth (root, shoot, leaves) and increased plant yield [110].
The paper of Takaki et al. described various CAPP treatments to keep the freshness and quality of agricultural products in the postharvest stage. For example, removing ethylene during storage and transportation (fruits and vegetables in storehouses, preservation boxes and transportation containers) is important because ethylene works as a plant hormone and it can induce fruit ripening and undesirable reactions (e.g., bitter flavors, yellowing of green leafy vegetables and increase of vulnerability to disease. Decomposition of ethylene is an oxidation process by atomic oxygen, which can be achieved using CAPP treatment (e.g., corona and DBD discharges) [111].
Plasma and plasma-treated water can also be utilized for controlling plant diseases (e.g., seed-borne, foliage, root and postharvest diseases) by inactivating pathogens (e.g., disinfection of seeds, fertilizer, water, soil) [23][109][112][113]. Review paper of Attri et al. concluded that plasma can also enhance seed germination (e.g., radish sprouts, wheat, sunflower, pea, tomato seeds). They described the possible mechanisms of plasma treatment in agriculture (increased gibberellin level and decreased abscisic acid content, enhanced activity of catalase, superoxide dismutase and peroxidase, improved water absorption, elevated levels of proline, chlorophyll, polyphenols sugar and protein contents) [114]. Seed germination efficiency (speed, percentage) depends on the plasma source, plant species and moisture content. Plasma-induced physicochemical changes in the properties of the seed coat or surface (e.g., elevated hydrophilicity and water permeability) enhance water imbibition, which is essential for seed germination. Adhikari et al. also discussed the beneficial effects of plasma treatment on seed germination, plant growth, and development. RONS can act as signaling molecules, which initiate a germination process and break the dormancy stage [108]. Another critical phase for plant development is the vegetative growth (it determines the overall crop productivity), which can be also regulated using plasma. As indicated by several studies, RONS have a positive effect on plant organs (shoots, roots, leaves and flowers) at different growth stages. Reactive species (H2O2 and NO) may disturb redox homeostasis and trigger mild oxidative stress in plants [108].
Another application of CAPP technology is the enhancement of seedling growth, which depends on seed metabolism and external environmental factors. Even 1 min CAPP treatment significantly promoted the seedling growth (fresh weight and length increment) of Arabidopsis thaliana. The results of Wang et al. suggested the plasma treatment accelerates the seedling abscisic acid (ABA) accumulations at the early stages of growth. ABA regulates the concentration of calcium (Ca2+) and RONS (e.g., OH, H2O2, NO−3, NO−2). The reactive species are easily transported through the cell membrane by diffusion and aquaporins. RONS serve as nutrients and also act as signalling molecules involved in the growth process [115].
Kučerová et al. studied the effect of PAW (produced form tap water using self-pulsing transient spark discharge) irrigation on lettuce plants. They concluded that H2O2 and NO−3 are the most important RONS in the PAW. However, proper concentrations must be applied in order to stimulate the growth process and positively affect the physiological parameters (number and quality of leaves, fresh and dry weight of plants, photosynthetic pigment content, photosynthetic rate and activity of antioxidant enzymes) of plants [116].
The study of Hashizume et al. represents the first trial of CAPP treatment in an actual production paddy field. They treated rice plants either directly by direct irradiation, or indirectly by immersing plants in a plasma-activated Ringer’s lactate solution (PAL). Their results suggest that the plasma treatment of rice seedlings is effective at improving plant growth, grain yield and grain quality. For example, direct irradiation experiments (plants irradiated during the vegetative growth period) resulted in increased grain yield by as much as 15% [117].
Pesticide (e.g., insecticides, fungicides, rodenticides, herbicides, garden chemicals) residues are toxic to human health and the environment. They have been linked to various illnesses, such as cancer, reproductive disorders and endocrine-system dysfunctions. It is therefore very important to focus attention on this issue and it is widely agreed that the use of pesticides should be regulated worldwide. The Environmental Working Group (EWG) annually releases the dirty dozen food list, which contains fruits and vegetables with highest traces of pesticides [118]. Results of Ali et al. confirmed that plasma activated water (PAW) and buffer solution were able to reduce (p < 0.05) chlorothalonil fungicide and thiram on tomato. Notable negative impact on the quality of the fruit was not observable based on pH value, ascorbic acid, titratable acidity, total soluble solid, lycopene and total phenolic content evaluations. The PAW increased the formation of RONS, which were beneficial to the degradation of various organic and inorganic pesticides [119].
Volkov et al. demonstrated that cold plasma (using plasma jet) can behave as a catalyst for important redox chemical reactions, such as the oxidation of nitrogen, occurring at the plasma/water and plasma/air interfaces, and in the volume of the liquid phase. Due to the reduced activation energy, atmospheric nitrogen can be converted to other forms (e.g., HNO3) useful in the agriculture industry for nitrogen fixation, production of nitrogen compounds and fertilizers. Plasma treatment of water may provide a promising alternative to current methods (e.g., Birkeland–Eyde, Haber–Bosch and Ostwald processes) which have well-known environmental and ecological issues [120].
Another new approach in plant disease control is the so-called plasma vaccination (RONS enter the plant through wounding or small openings and further penetrate the plant cells), which activate plant immune response [109].
Plant growth in space is under research as a possible technology to be used in future. Plants provide fresh food, oxygen and psychological benefits for astronauts on long -term missions. CAPP as a waterless, chemical-free technology has potential as a disinfection tool in spaceflight, however even stricter regulations are needed [25].
Another branch of agriculture is animal husbandry dealing with animals (e.g., cattle, sheep, goats, pigs, chickens, rabbits and insects) raised for meat, milk, eggs and fiber. Good animal husbandry practices are essential for animal welfare and productivity. CAPP can be helpful in maintaining better health conditions of animals via i) microbial decontamination of air (deactivation of indoor and outdoor bioaerosols), water (E.coli), food, instruments (surface pathogens, such as methicillin-resistant staphylococcus aureus, Klebsiella pneumoniae); ii) wound healing in animals (e.g., wound myiasis caused by blowfly, inactivation of Chlamydia trachomatis); iii) packaging of animal products (sterilization of packages, disinfection the inside of sealed bags) [121].
Newcastle disease and avian influenza are the two most common devastating diseases among poultry (chicken, turkey and duck). It has been already reported that CAPP can be used as an effective and safe agent for inactivated vaccine preparation against viruses [121]. The study of Su et al. indicates that virulent Newcastle disease can be inactivated using plasma-activated solutions (H2O, NaCl, H2O2). The efficiency of the inactivation was confirmed using embryo lethality assay and hemagglutination tests. This disease affects bird species and has a large economic impact on the domestic animal and poultry industries; furthermore, it is transmissible to humans [122].
Plasma-based inactivation of prions (proteinaceous infectious particles), which belong to one of the most resistant pathogens, is also under research. Most prion diseases affect the nervous system of humans (Creutzfeldt–Jakob disease) and animals (bovine spongiform encephalopathy in cattle, chronic wasting disease in cervids). Prion diseases have long and silent incubation periods, once the symptoms emerge, these disorders are rapidly progressive and usually fatal [16].
Key benefits of utilizing CAPP in the food and agriculture sectors include minimal water and power usage, operation at ambient temperature, and short treatment duration (from seconds to minutes). Furthermore, its use is free of hazardous solvents, it reduces preservative use and it is applicable for both solid as well as liquid phases [10][16]. Currently available data confirm that CAPP technology could be utilized in the agriculture and food industry [22][99]. To achieve broad applicability, an efficient open-air device suitable for the treatment of both large areas and high number of samples is necessary [16]. An ideal industrial scale device allows homogenous disinfection of various products during the sorting process on rolling electrodes [23].
It is important to avoid quality degradation or any undesired effects of plasma treatment. In addition, more intense optimization studies are needed to fully understand the mechanisms behind the pathogen–plasma interactions. There is a need for standardization of the plasma dose (treatment duration is not an appropriate standardized unit because of the diversity of food products and plasma reactors) [11][25]. CAPP treatment limitations are also necessary to account for before consumer acceptance can be achieved [10][11][109].
As claimed above, CAPP produces different reactive species (RONS, positive and negative ions, atoms, molecules, etc.) playing a crucial role in the inactivation of microbial targets. Bourke et al. reviewed the mechanism of bacteria inactivation via CAPP. The bacteria are eroded by cell bombardment of charged particles, breaking the appropriate chemical bonds and opening the cell membrane to the penetration of reactive agents into the inner volume of bacteria. The etching process (facilitated, in particular, by RONS species) subsequently causes formation of molecular fragments leading to morphological changes of the cell (e.g., formation of deep channels in the bacteria), RONS cause damage by oxidation of cytoplasmic membrane, protein and DNA and thus complete bacterial destruction [57]. To increase the use of CAPP technology in industry, it is important to provide theoretical studies about the pathogen inactivation process. To describe the inactivation mechanism at the atomic level is difficult based on only experimental research [123]. A better understanding of plasma dynamics and chemistry, and the transport of reactive species in the plasma can be achieved using numerical simulations. Most computational studies evaluate the plasma physics and gas phase chemistry (e.g., spatial-temporal profile of the electric field, density of reactive species, radiation intensity). Various kinetic models (classical reactive molecular dynamics, nonreactive molecular dynamics, density functional theory) are used to describe the biomolecular systems at the molecular or atomic scale [124]. These models save time, money and other resources by analyzing a large amount of data at the same time and provide valuable correlations. The study of Cui et al., based on reactive force field molecular dynamics simulation, found that ROS (O, OH, HO2 and H2O2) reacted with the S. cerevisiae glucan structure by hydrogen abstraction reaction to cleave the chemical bonds (C–O and C–C), which resulted in cell wall destruction. Their results showed that O (highest activity) and OH induced the largest number of hydrogen abstraction reactions. The activity of HO2 and H2O2 were lower, however, the number of main chain and branched chain fractures were bigger. Consequently, the destructive effect of H2O and H2O2 was more efficient [123].
The bacterial cell membranes, barriers protecting proteins and nucleic acids can block ROS from entering the interior of cells. However, ROS with high permeability are able to enter the cell interior and cause cell death. Therefore, the permeability of ROS into the bacterial membrane plays a crucial role in the bacterial inactivation mechanism [125] as was shown by Hu et al. Their molecular dynamics simulation described the behavior of various ROS at the membrane–water interface. They showed that the cell membrane acts as a weak barrier to O2 (hydrophobic ROS), which remains within the lipid bilayer, and plays the most important role in oxidation reactions. On the other hand, the cell membrane serves as a strong barrier to protect internal substances from the hydrophilic ROS (OH, HO2, H2O2). The permeability of the cell membrane to HO2 was markedly enhanced after the CAPP treatment, which enhanced the bacteria-killing properties of the plasma. They also concluded that the arrangement of phospholipid molecules (the main constituent of the cell membrane having hydrophilic head and hydrophilic tail) in the cell depends on the ROS generated by the plasma. High amount of ROS resulted in decreased cell membrane thickness and disordered phospholipid arrangements [125].