1000/1000
Hot
Most Recent

Plasma technology water purification is a new water treatment technology developed according to the trend of industrial water use in the 21st century. It is effective, efficient, scalable, versatile and customizable. These technologies must be able to adapt to new contaminants, reduce energy consumption, maintain or improve the proportionality between power and flow, demonstrate various flow capacities, minimize the transformation of existing infrastructure, prepare for imminent regulations, and tailor chemistry to site-specific requirements. New methods of water treatment by plasma must have all the above-mentioned properties and pose the least risk to public health. NTAPPs and their chemical reactions release energy and reactive chemical species that can kill bacteria and microorganisms, resulting in the disinfection of water. The advantage of this technique is that it can be performed in ambient air under atmospheric pressure without a vacuum system. Furthermore, NTAPP does not involve chemical products such as Cl. NTAPP can be used for water treatment in three ways: via direct, indirect, and bubbling methods.
Non-thermal plasmas have been achieved under normal atmospheric pressure by avoiding gas heating with the help of dielectric barrier discharge (DBD) [1][2]. DBD has been performed with the help of a strong electric field [3][4][5]. The source structure for a plasma discharge system consists of two electrodes, where either or both are concealed by a dielectric layer. Figure 1 depicts various DBD plasma sources. DBD has an electron density in the range of 108–1015 cm−3, an electron temperature in the range of 1–10 eV, a gas temperature of approximately room temperature, and a plasma dissipation energy of approximately 1–10 W [6]. When the breakdown voltage is achieved, luminous DBD is produced at atmospheric pressure when neutral gas is ionized after the collision of electrons with the working gas [6]. A distance between electrodes, flow rates, and nature of working gas (mainly that of noble gases such as He or Ar) determines the magnitude of applied voltage. NTAAP can be supplied by direct, pulsed direct, or high frequency alternating currents (radiofrequency and microwave). Interaction between the NTAAP and air can be minimized by using purging gas or shielding. The main feature of DBD is the existence of dielectric material along with the electrode. Gas is ionized above the dielectric surface in accordance with the electric field. The working frequency range of DBD is generally 50–500 kHz, and the voltage is approximately 1–30 kV [4][5]. DBD plasma or plasma jets are generally selected for water purification [7], bio-sterilization [8], decontamination [8], and biological applications.
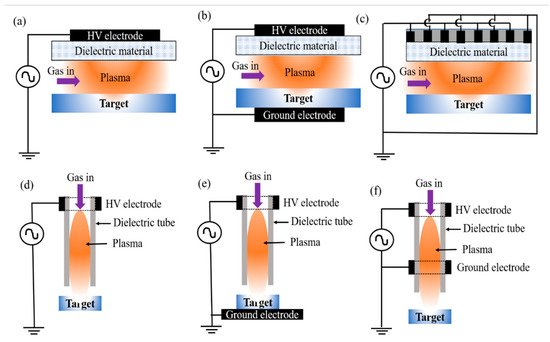
Figure 1. Schematic diagrams of DBD plasma sources: (a) floating electrode DBD plasma source, (b) facing electrode DBD plasma source, (c) surface DBD plasma source, (d) floating electrode plasma jet, (e) direct plasma jet, and (f) indirect plasma jet treating a sample.
Figure 1a–c provide schematics of surface DBD, specifically, a floating electrode DBD plasma source, facing electrode DBD plasma source, and surface DBD plasma source, respectively. It consists of two parallel plates in a planar or facing pattern. The electrode separation in a DBD is made to be in the range of micrometers to a few centimeters. Plasma is formed in a non-uniform electric field. In DBD, the electrode gap can be changed according to the application requirements. DBD plasma is applicable for large area surface treatment. Figure 2d–f illustrate the use of a volumetric luminous plasma jet formed by a dielectric tube and one or two electrodes. The plasma is produced inside the dielectric tube and then appears in the ambient air along with the gas flow. In all types of sources, plasma is produced within the high voltage and ground electrodes, which are separated by a dielectric tube. Plasma jets are improved designs of DBD plasma sources whose plasma plumes elongate to the order of a few centimeters. The plasma plume ejected from a discharge tube into an open space looks like a continuous luminous plume to human eyes; however, it propagates discontinuously as a plasma bullet [9]. Reactive species, electrons, and ions generated by the plasma discharge are delivered to the target via the dielectric tube and ambient air. The differences between plasma jets mainly originate from variations of the electrode, electrode material, dielectric material, working gas, frequency, applied voltage, and electrode separation. In this regard, Reuter et al. [10] and Ito et al. [11] described the control of RONS production in liquid by NTAPP with different shielding gases. Ghimire et al. [12][13] discussed the variation of RONS with the gap distance, electrode separation, and plasma propagation length. Yue et al. [14] and Lamichhane et al. [15] studied the effects of working gas on the concentration of RONS. Similarly, Lim et al. [16] and Nguyen et al. [17] presented the effects of the applied voltage, frequency, and moisture on atmospheric pressure plasma.
As mentioned above, NTAPP generates various reactive oxygen species (ROS), such as OH radicals, O2−, H2O2, O, O3, 1O2, and reactive N species (RNS), including NO, NO2, NO2, N2O5, and atomic N [18]. Some of them are short-lived, such as OH radicals, O, NO, N, and 1O2 [19]. The RONS are formed due to the energy associated with the collisions between accelerating electron and neutrals and are referred as primary reactive species. Electrons (e−), ionized neutrals and gas (M+), excited neutrals and gas (M*), N, O, atomic H, NO, and O2*– are formed in the gas phase immediately after the collision [20][16]. These species are called primary reactive species [21], and their intensities within the plasma region are very high. Primary reactive species have very short lifetimes; for example, the lifetimes of OH radicals, NO, and O2*− are ~2.7 μs ~1.2 μs, 1.4 μs, and ~1.3 μs, respectively [22]. Some of these reactive species immediately undergo radiative decay, and others are combined with other reactive species, neutrals, and water molecules. The primary reactive species are transformed into other reactive species such as H2O2, NO2, NO3, and O3 [23] in the ambient environment to form secondary reactive species [21]. The RONS produced in the gas phase reach the liquid phase (or another target) and are dissolved, forming tertiary reactive species [21]. O3, H2O2, NO3−, and NO2− are long-lived reactive species because they last for a few milliseconds to several days [24]. H2O2, NO2, and NO3 are soluble in water, and NO2 and NO3 are immediately changed into NO2− and NO3−, respectively [20][25]. When these reactive species dissolve in liquid, the pH of the target liquid decreases by up to 2 depending upon the plasma source, working gas, power source, treatment time, and sample volume [25]. However, the chemistry of ROS and RNS formation in a target differs depending on whether the target is dry or aqueous [25]. Figure 2 provides a schematic diagram of RONS formation within the discharge region, gas region, plasma/target interface, and inside the target.
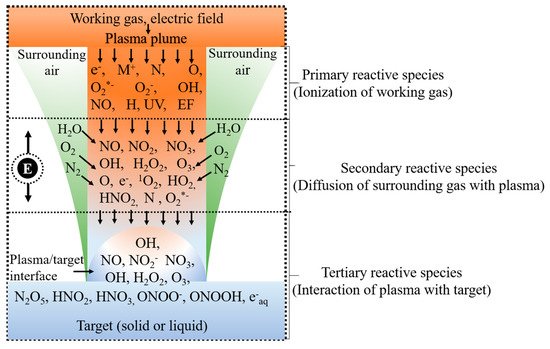
Figure 2. Schematic diagram representing the formation of various reactive species in the plasma phase, in the gas phase, in the interfacial region, and below the interfacial region during plasma irradiation of a target.
As mentioned above, for purification, bio-sterilization, and decontamination of water, OH radicals, H2O2, and O3 play vital roles. These reactive species are formed in the plasma and plasma–liquid interface through numerous possible pathways. The main reaction pathways are explained in this section. When plasma is in contact with water, OH radicals are produced via chemical and physical processes. They can be in either aqueous or gaseous form. OH radicals can be formed by the dissociation of water via electron collisions, UV photolysis, and collisions with metastable or excited particles. The pathways of OH radicals consumption are even more complicated in discharges of high energy density due to the complexity of water dissociation and fast aqueous reactions [22] via Equations (1)–(6):
In plasma–liquid interface, the penning ionization is also possible, as in Equations (3) and (4) [19]:
Form UV photolysis of water, it can be achieved from Equation (5) and (6) [26]:
H2O2 is an important biologically reactive molecule involved in bacterium inactivation and cell oxidation stress [27]. Chemically, H2O2 is a ROS with 1.8 V oxidation potential. H2O2 are produced in plasma from the recombination of two hydroxyl radicals in the liquid or plasma phase [28]. In the plasma phase, H2O2 can also be generated by the interaction between excited water molecules and hydroxyl radicals as shown in Equations (7)–(9) [22][29]:
Similarly, ozone is another plasma product to give the oxidative stress. Plasma electron can disassociate O2 into atomic O. This mechanism are the main conditions for the occurrence of tree reaction bodies in the formation of O3 via Equation (10) [14]. In this reaction, M represents other molecules inside the reactor, such as N2, O2, Ar, He, and Ni.
Energy consumption estimation is another crucial step before real-life application of any technology. Nguyen et al. [17] studied the H2O2 concentration according to the total energy for various applied voltages and frequencies in underwater plasma discharge [17]. They found that the frequency source has vital effect on the total energy consumption. According to their estimation, underwater plasma discharge consumes a few tens of kilojoules per millimole to 100 kJ/mM H2O2. The production rate versus energy consumption graph is almost linear, and the coefficient is approximately 0.12 mM/kJ for different cases, as mentioned in [17]. Figure 3 depicts the variation of the O3 synthesis efficiency with the electric field strength (E/n) in DBD from [30]. Where solid line represents the trend of scatter plot (*) of energy efficiency against the electric field. The O3 synthesis efficiency depends on the electric field strength, and the optimum efficiency is approximately 225 g/kWh at 200 Td.
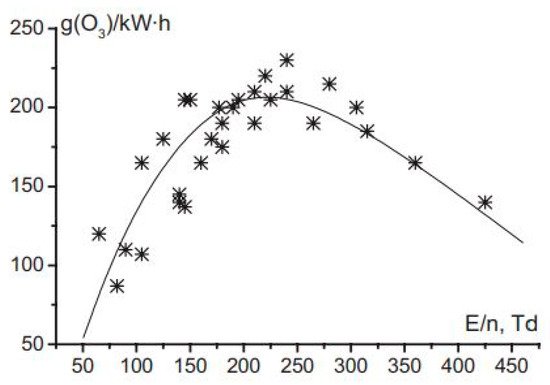
Figure 3. Variation of energy efficiency for ozone synthesis with electric field strength in DBD (Reprinted from [30]).
Plasma chemistry can be used to decompose the various chemical and biological pollutants. Recently Takeuchi et al. [31] summarize the plasma enhanced water treatment technology to decompose the organic compound present in water. A graphical representation of breakdown of total organic compounds (TOC), denoted by R, with oxygen plasma at plasma–liquid interface is shown in Figure 4a [31]. The breakdown of TOC is only possible about the plasma–liquid interface, where OH radicals, H2O2, O3 is abundantly available. In their report, energy efficiency for breakdown of TOC in wastewater was about 0.22–0.29 g kWh−1 and breakdown rate were about 6.0–7.3 mg h−1 [31]. The TOC decomposition efficiency rate achieved by several methods including chemical, plasma, and synergistic effect of plasma and ozonizer are compared in Figure 5b [31]. A synergistic effect of plasma and ozonizer has reasonably high efficiency as compared to conventional AOPs with high organic compound breakdown rates [31]. Among the different plasma sources nanosecond-pulsed discharge plasma with water droplets reaches an elevated TOC breakdown rate and efficiency [32].
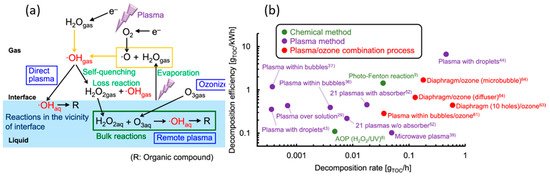
Figure 4. (a) Graphical representation of main reactions involves in the breakdown of organic compound using oxygen plasma in plasma–liquid interface. (b) Comparison between the breakdown rates and efficiencies of organic compound with several methods. (Figure (a) and (b) are reprinted from [31]).
Traditional water treatment methods cannot remove various pollutants such as halogenated hydrocarbons, aromatic compounds, pentachlorophenol, pesticides, herbicides, and more recently pharmaceuticals. The effective decontamination of wastewater and unpurified water from natural sources is a major social concern [33]. The conventional strategies for water filtration and sanitization, although successful at eliminating numerous toxins, are less capable of absolute purification. Recent exploration has revealed the presence and risky nature of destructive natural mixtures present in many water supplies, which cannot be eliminated by customary decontamination methodologies [34]. These contaminants include endocrine-disrupting compounds and pharmaceutical drugs as well as by-products of manufacturing processes and certain pathogenic microorganisms, which are robust against filtration and chlorination and are directly correlated with human health, causing hormone disruption, cancers, and birth defects [33][34]. Among these substances are volatile organic compounds, which can become stuck in air and water and thus can pollute surface and groundwater resources and threaten the drinking water supply.
This concern regarding the tainting of our water supply demands the presentation of new, productive, and viable water purging technologies capable of eliminating these contaminants. To check for these pollutants, innovations and techniques that completely remove water pollution will be necessary. NTAPP discharges have recently been used to resolve this issue. Plasma reactions can occur in wastewater via two mechanisms: plasma formation in contact with the liquid surface and production directly within the liquid. In the first mechanism, the plasma interacts directly with the liquid surface at the liquid–gas interface. The chemical reactivity in plasma is evolved using the interface reactions and transport of reaction species from the gas phase to the liquid phase. The second mechanism can be implemented via the propagation of streamer discharge either within bubbles generated by external gas injection or in microbubbles produced by the field [35]. NTAPP is a source of charged particles, radicals, excited and reactive species, shockwaves, ultrasound, and UV radiation, which are not generally harmful to health or the environment. These species can significantly inactivate bacteria and destroy viral infections by degrading organic molecules. In recent years, different kinds of plasma with various reactor geometries have been studied for water treatment applications. It has been shown that a straightforward gas-phase discharge in contact with liquid water is better than breaking the discharge using submerged electrodes in the water. In 2017, Sukhwal et al. [35] designed a micro discharge plasma jet (MDPJ) with a simple geometry and low power consumption. Their discharge system consisted of a high voltage power supplier, high voltage electrode, grounded electrode, dielectric tube, and developed reactor. The authors showed that the stable production of plasma and active species such as RONS is key for water purification using an MDPJ. When a gas enters the inner electrode, the gas velocity increases due to the increasing gas flow rate, and consequently, the length of the plasma jet increases as well [35].
The reactive species produced by plasma jets, such as O, H2O2, OH radicals, NO, and NO2, offer strong sterilization of Escherichia coli and thereby contribute to water purification [36]. Even a low amount of H2O2 can be used as a strong oxidizing agent for pathogen sterilization. Nitric and nitrous acid, which are generated by NO2 and NO, are the main chemical species that cause acidification of the treated fluid. The concentrations of these main reactive species, such as those of NO2 and H2O2, are proportional to the length of the plasma jet because a larger interface between the water and plasma jet enables more reactions for oxidizing agent production. In 2018, Suraj et al. [36] designed a water purifier based on plasma technology. They showed that the optimized gas flow rate was 4 L/min based on the concentrations of NO2, NO, and H2O2 generated. Pathogens were eliminated from the water by exposing a prototype to the plasma stream and its UV radiation. To convert the polluted water into a gas–liquid mixture, the authors used a water pump that accelerated the polluted water to high speeds. Finally, to obtain the liquid form of clean water, the mixture was slowed down. The authors showed that compared with other wastewater treatment methods, this new plasma–water treatment system was more efficient and cheaper. Block diagram of plasma-based water treatment method and photograph of water treatment plant is shown in Figure 5.
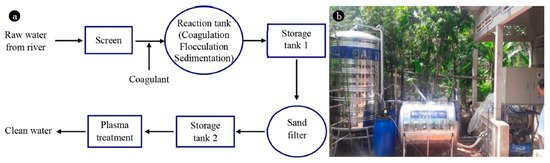
Figure 5. (a) Block diagram of water treatment and (b) water treatment with NTAPP (reprinted from [37]).
When the plasma touches the solution, its interaction with the solution can lead to two types of electron-induced reactions. One is between the accelerated electrons and a very thin layer on the liquid surface. This process can lead to dissociative electron attachment. The second is related to the aqueous electrons that do not participate in reactions near the liquid surface and can produce OH− and H. It is worth noting that the reaction rate can be found to depend on the discharge parameters, such as the discharge current. Both the electron density (and flux) and energy distribution of electrons in the plasma are related to the discharge current, as indicated by the following relation as shown in Equation (11) [38][39]:
where ne is the electron density, j is the discharge current density (current per unit area), E is the electric field, and μ is the electron mobility. When the discharge current increases, the electron density in the plasma increases, and the ratio of the reactive species produced by the plasma in the aqueous solution consequently increases as well. These results imply that increasing the discharge current in the plasma could enable acceleration of the electron transfer rate.
Another water treatment technique involves use of O3 plasma. In 2019, Ali et al. [40] investigated the effects of this type of plasma on peat water. Injecting O gas into an area with a high voltage produces O3 plasma. This plasma can initiate different mechanisms for purifying water. Passing through the corona plasma unit will produce O3 plasma. By passing the O3 through a charged tube with water in the presence of internal high stresses, an oxidizing compound such as H2O2 as a hydroxide radical is produced. This mechanism will produce more effective OH radicals, O3 and H2O2 that have an oxidation ability that can be used to destroy germs and decompose materials that cannot be degraded by conventional methods. The used O3 and OH radicals will break down into H2O and O2. These particles will be released as treated water with high dissolved O content and clarity that meets clean water standards. Compared with the other conventional methods, the plasma and liquid interaction produces more oxidizers that affect the oxidation level. The characteristics of this approach are as follows: (1) as ordinary air can produce O3 plasma, additional consumables are not needed; (2) the corresponding organic compounds decompose relatively effectively; (3) as consumables are not needed, the costs of and infrastructure for consumables can be neglected; and (4) O3 plasma can be used as final stage in water treatment because it is inherently modular. O3 plasma also can be employed for the elimination of micro contaminants. Different techniques can be used for color removal, such as oxidation–reduction, coagulation–flocculation–sedimentation–filtration, and the use of activated C or adsorption–absorption. These processes have important effects on the water treatment cost and require additional media or chemical compounds. Importantly, Ali et al. [40] showed that dissolving O3 and OH radicals in the water by maximizing the chemical processes occurring in the O3 plasma system can remove color without additional chemical media. It has been proven that O3 plasma can treat peat water by reducing the color level in the water [40].
An additional review by Foster et al. [41] presented an overview of the limitations and future of advanced oxidation technology using plasma for water treatment. They stated that plasma is produced on the liquid surface due to the liquid–gas interactions. This production method can be performed in different ways, including through glow discharge electrolysis, DBD, and gliding arc discharge. Due to the ability of plasmas to undergo AOPs, this technology has been considered for the decomposition of organic-compound-polluted water. Kim et al. [42] reported the effects of spark plasma discharge on sulfate-reducing bacteria and the inactivation of acid-producing bacteria in water and described the residual effects of plasma treatment on bacterial inactivation. They investigated both clear and dark produced water. The cost of energy for 1–log reduction of acid-producing bacteria in both clear and dark waters was 1.5 kJ/L in the flow rate range of 0.063–0.315 L/s used in the flow tests. Their work stated that the energy efficiency of bacterium inactivation in clear water depends on the type of plasma. Meanwhile, Barillas et al. [43] used a prototype of plasma technology for water purification and indicated that it could decrease water source contamination mainly due to sewage spill by the industrial sector. To remove pathogens from contaminated water, the prototype converted the liquid water into plasma. The properties in plasma leading to pollutant destruction are UV radiation, shock waves, and electric fields. The plasma used in this work was obtained by applying an AC power supply to high voltage electrodes.
Further new technology for water treatment, as the first kind of direct plasma system that produces plasma in contact with water, is corona discharge [44]. This technology employs plasma discharge to generate RONS in a powerful and efficient manner. The solution is chemical free, using electric energy to generate strong oxidants from the air and water itself. Therefore, it can readily destroy dissolved organic pollutants in water while effectively removing color and odor. Creating an economical alternative to conventional ozonation was the original motivation for using plasma directly in water treatment. The main idea was to generate oxidant species at the right positions in the water and use the power of OH radicals. The formation of such species is a considerable benefit. This technology has advantages such as that the solution is chemical free, and that electric energy is used to generate strong oxidants from the air and water itself. In addition, unlike UV-based approaches, the process is insensitive to turbidity, and unlike chlorine and peroxide techniques, corona discharge has no need for chemical logistics, storage, or usage.
As mentioned earlier, conventional water treatment methods cannot satisfactorily remove all contaminants. For instance, species that are resistant to Cl (e.g., bacteria spores and protozoa) are not eliminated by conventional methods, although AOPs can be used with traditional techniques such as filtration to decompose these contaminants. Furthermore, NTAPP species (charged species, reactive species, and UV photons) have significant effects on sterilization and microorganism disinfection. Previous studies have demonstrated that the interaction between plasma and water can produce H2O2, which has wide scope to disinfect the various microorganisms such as viruses, molds, and bacteria. It is worth noting that some chemical and physical parameters of the liquid (e.g., overall hardness and total dissolved solid, and conductivity) change slightly due to interaction with the plasma [37]. Substantial research has been conducted in this area in recent years. The present article reviews numerous research papers concerning the interaction of plasma with wastewater to inactivate bacteria.
Zhang et al. [45] used an AC-driven microplasma jet array under atmospheric pressure with a repetition frequency of several kilohertz to inactivate resistant Pseudomonas sp. HB1 cells in water. The results showed that the species with short lifetimes (such as charged particles and OH radicals) in microplasma jets could effectively inactivate resistant Pseudomonas sp. cells. With 108 colony-forming units (CFUs) in an 80 mL suspension, all Pseudomonas sp. cells were killed within 6 min of plasma remedy. Various studies have been conducted on the elimination of Escherichia coli in wastewater using NTAPP. Similarly, Ziuzina et al. [46] considered the effects of cold plasma produced in a closed chamber on Escherichia coli inactivation under atmospheric pressure. All samples in this research were directly and indirectly treated with plasma, and the impacts of treatment and post-treatment time were investigated. The results demonstrated that this plasma configuration can eliminate high concentrations of Escherichia coli in water in several seconds. An important benefit of this approach is the absence of post-processing pollution. Plasma treatment directly leads to the complete inactivation of bacteria. Meanwhile, Zheng et al. [47] used water-pulsed NTAPP for water disinfection (Escherichia coli inactivation). Their results indicated that the liquid conductivity had a significant role in the creation of plasma containing reactive species and UV emission and revealed its effect on disinfection. The disinfection effect was dramatic when the conductivity of water was up to 1.5 mS/cm. This study indicated that during water sterilization, OH radicals has strong chemical sterilizing power. However, because of the short lifetime of OH radicals, which is only a few milliseconds, it is difficult to use OH radicals to disinfect pathogenic microbes directly. Hangbo et al. [48] investigated the effects of various ROS with short lifetimes on yeast cell inactivation in a liquid and showed that 1O2, OH radicals, and O2− are the three main particles created in a plasma–liquid system. Based on the experimental results, OH radicals has the least contribution to plasma inactivation among the plasma species. In contrast, O2− and plasma acid play major roles in inactivation. It is worth noting that in the plasma–liquid interaction, 1O2 is a more significant antimicrobial factor than the species mentioned earlier (i.e., OH radicals and O2−). It should be noted that despite the strong oxidizing properties of OH radicals, it does not have the capacity for significant diffusion [48]. Because of its short life and high reactivity, it can only react with nearby particles. On the contrary, 1O2 can effectively diffuse into the cell layer and initiate peroxidation of lipid due to its lifetime being longer than that of OH. Moreover, the results illustrated that in plasma devices (e.g., DBD plasma and plasma micro jets), the 1O2 concentration is often greater than the OH radicals concentration. Nevertheless, O2− requires a greater oxidation ability to remove H2 from the phospholipids and consequently can only enter the cell hydrophobic area with significant effort. However, by the Haber–Weiss reaction [49], O2− can be changed into OH radicals and converted into hydroperoxyl (HOO) radicals by protonation when the pH is less than 4.7. Hence, these species can easily enter the cell hydrophobic area. Moreover, it has been demonstrated that plasma acid is a significant factor in the inactivation of plasma–liquid media. It is a vital parameter in enabling plasma species to pass into cell walls and in decreasing the resistance of cells versus acidic media [50][51][52][53]. To enhance the effects of sterilization, it is important to develop a device in which most components in the liquid (microorganisms) could come into direct contact with the plasma. The results showed that NTAPP performs sterilization much more effectively, because the concentrations of RONS causing sterilization are quite high in NTAPP, and the probability of reaction between Escherichia coli and the plasma-generated RONS increases [35]. Both nitrous and nitric acid are formed through the interaction of NO and NO2 with water by hydrolysis. Meanwhile, NOx is produced by reaction with the air, but when plasma interacts with liquid and thus does not have the opportunity to contact air, NOx generation barely occurs. Furthermore, air plasma jet have a greater sterilization effect than nitrogen plasma jet due to the generally huge number of reactive species causing bacterial inactivation [35]. Moreover, the radical concentration affects the sterilization in proportion to the plasma length (e.g., it increases with increasing gas flow rate). In another study [44], the behavior of the surface potential of bacteria was investigated as a function of the applied energy by analyzing the molecular levels of DNA and proteins. The applied pulse voltage (23 kV) and frequency (25 Hz) in the experiments enabled rapid disinfection. In a treatment time of 6 min, complete disinfection was achieved. This result indicated that plasma could increase the protein leakage via the membrane of bacteria. It is interesting that in this case, approximately 70% of all proteins were leaked during the first 8 min of treatment.
The plasma spark method is another important approach for water disinfection. Rashmei et al. [54] investigated water disinfection (Escherichia coli and Enterococcus faecalis inactivation) using the plasma spark technique and compared the results with those of ordinary methods. In this research, some of the chemical and physical parameters of the water were evaluated. The results demonstrated that Escherichia faecalis and Escherichia coli decrease by 8 log CFU after 12 and 15 min, respectively. Moreover, this research showed that major bacteria inactivation in water is achievable using electric fields and H2O2 molecules produced using plasma. NTAPP also has a powerful ability to remove bacteria. A concentration reduction of 8 log CFU was observed in all samples. In this method, some plasma species (e.g., H2O2, OH radicals, O3) are strong oxidizers with the necessary ingredients of bacterial invasions, including lipids, proteins, and DNA, as summarized in Figure 6.
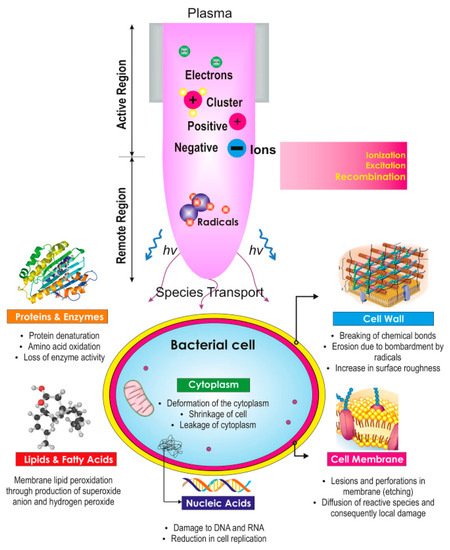
Figure 6. Action of NTAPP on bacterial sterilization (reprinted from [55], Copyright Elsevier (2017)).
After applying plasma to drinking or wastewater, the effects of different types of organic and inorganic matter on the disinfection process have been investigated. The results have shown that the concentrations of inorganic and organic compounds in tap water can change after treatment [25]. It is worth noting that these changes are ascribed to the material interaction of the plasma species in tap water. It is advantageous that NTAAP can dramatically change the organoleptic characteristics of the water, such as smell, while increasing the amount of soluble O and decreasing the required concentration. However, plasma dramatically increases the concentrations of NO2 and NO3 and can significantly enhance the conductivity and decrease the pH [37]. On the other hand, cold plasma also has a negative effect on turbidity. Thus, the turbidity increases after treatment, because by using O3 dispersion with a porous plate, large particles can be broken into many small ones [37]. Furthermore, plasma significantly enhances the concentrations of NO2 and NO3 due to the existence of N in air in the plasma formation zone. Thus, plasma technology could solve the existing drawback in conventional water sterilization and disinfection method using the exchange resin of the anion. To design a plasma device, parameters such as the device output, device lifetime, operation volume, known toxicities of the ingredients, and pre- and post-remedy demands should be considered [56]. One of the major benefits of increasing the scale of plasma devices from the laboratory scale to the industrial scale is that the size of the of plasma–liquid interface can be increased [57]. The most effective devices for water treatment are those that expose thin layers of the liquid to plasma because the plasma–liquid interface determines the treatable throughput. These configurations increase the plasma induced in the water as much as possible. Reactors with high throughput are convenient for practical applications.
The problem of surface and ground water pollution with toxic chemicals is a significant challenge globally [58]. Plasma-generated RONS has been widely used to remove many toxic chemicals, including organic compounds, phenol, organic dyes, and pesticides [58]. Among the available plasma sources, NTAAP can eliminate toxins in polluted water without forming any secondary contaminants, making it a practical choice to solve this challenge [47]. Hijosa-Valseroa et al. [58] described the fabrication of a DBD plasma device at atmospheric pressure to eliminate harmful toxins in liquid media. The destruction of bisphenol A and tributyltin in distilled water using the DBD reactor was demonstrated. The use of DBD enables plasma to operate with AC for ignition. It is normal for the elimination efficiency to decrease in actual wastewater treatment. However, DBD plasma reactor treatment provides fast elimination without external species such as O3 and H2O2 or pH adjustment. DBD plasmas have gained popularity in this regard due to their stability under ambient conditions, easy operation, and large discharge areas [59]. It has been shown that in this method, many chemical and physical (e.g., UV and shock wave formation) conditions can, either directly or indirectly, lead to the destruction of organic compounds, and the contributions of these agents are strongly related to the parameters of the discharge plasma.
Hu et al. [59] described the kinetics of dimethoate and dichlorvos destruction using a DBD device. The discharge power and DBD device structure significantly affected the destruction efficiency. The findings of this study indicated that a higher destruction efficiency can be achieved with a smaller gap distance and greater discharge power. Moreover, the authors showed that RONS in DBD reactors can have significant effects on pesticide degradation. Specifically, the destruction of organophosphate pesticide with different initial concentrations in water was investigated. The results indicated that with increasing initial concentration, the pesticide decomposition rate decreased. This finding could be of great significance for the development of a sewage remedy including organophosphate pesticides. In the wastewater treatment industry, saline dye wastewater treatment is one of the principal approaches. Xu et al. [60] reported the effects of various destruction parameters on saline and azo dye destruction in liquid media using glow discharge. The results illustrated that this technology could turn into a technology for treating saline dye wastewater. The results indicated that the electrolyte, initial C. I. Acid Red 73 (AR 73) concentration, and initial pH significantly affected the degradation of AR 73. The reduction of the parameters improved the AR 73 degradation results.
The existence of organic pollutants in water has caused substantial concern owing to the adverse effects on the environment and human beings. The elimination of pharmaceutical components in water using NTAPP has gained significant attention due to the occurrence of these contaminants in surface water and occasionally even in drinking water [61][62]. Industrial pollutants in contaminated groundwater generally release volatile organic complexes, such as m-xylene and toluene, into the neighboring regions [63]. In this regard, Abdullahi et al. [64] developed a method in which a combination of air stripping and used an NTAPP-based technique in the destruction of m-xylene and toluene in wastewater. To optimize the performance of the NTAPP reactor, the response surface methodology was applied to investigate the interactions between distinct parameters. The experimental model calculations and removal efficacy had errors of approximately 2.16% and 1.25% for m-xylene and toluene, respectively. This developed model could satisfactorily fit the experimental data [64]. In another study, phenol degradation was studied by plasma treatment. The phenol degradation results achieved by underwater plasma treatment were widely discussed in a recent review [65] mainly focused on their elimination strategy. NTAPP exposure degrades phenol liquids efficiently, where the resulting reaction products can be identified by high-performance liquid chromatography (HPLC). The mechanism of phenol oxidation by the presence of OH radicals and O3, as well as more detailed nitration and nitridation mechanisms, has been explained previously [66]. Numerous studies have indicated that compared to traditional AOPs, NTAPP treatment has the potential to degrade pharmaceuticals with high energy efficiency and low energy requirements compared to O3 treatment [67][68][69]. This property makes NTAPP application a practical substitute for conventional wastewater management processes. The primary benefits of using NTAPP to destroy organic composites include the minimal environmental harm caused by this approach. NTAPP-generated reactive species, such as OH radicals, H2O2, and O3, not only react superficially with pollutants, but also diffuse in the bulk phase [70][71][72]. Dimethyl phthalate (DMP) is widely used as a plasticizer and acts as a water pollutant. Qi et al. [73] used a micro plasma-based AOP process wherein liquid–gas discharge was used for DMP removal. Moreover, the use of this enhanced performance method was accomplished by comparison with the other existing methods, including electrocoagulation and ozonation. The authors observed that plasma-generated O3 and OH radicals were the key reactive components inside plasma-degraded DMP liquid, which hit numerous DMP functional groups for degradation. The effectiveness of DMP degradation is highly reliant on the treatment time and discharge power when used for water treatment. This report shows that air microplasma exposure could be used as a possible tool for wastewater purification [73].
Several pharmaceuticals have been observed in above-ground water [74] and groundwater [75], including streams [76]. Figure 7 depicts the application of NTAPP for pharmaceutical component degradation in water. Antibiotics, as one class of pharmaceuticals, are widely studied due to the development of bacteria with antibiotic resistance [77]. Their oxidative elimination by plasma is quite beneficial; nonetheless, mineralization has been confirmed to be moderately slow [68][78][79]. Based on the determined oxidation intermediates, researchers have suggested that the main degradation mechanism relies on OH radical attacks, subsequent hydroxylation, and the damaging of molecular bonds, which leads to mineralization [78][79]. Reports on the application of NTAPP to remove antibiotics such as atenolol [80], verapamil [81], and enalapril [82] have been published. Krishna et al. [81] proposed that DBD reactors attack OH radicals and O3 on the amino group and aromatic rings for verapamil oxidation. Another compound called carbamazepine, the highest determined environment pharmaceutical, is hazardous to aquatic species and has endocrine-disrupting activity [83]. To address this issue, Liu et al. [84] developed a low-power ex situ plasma treatment method, which exhibited high efficiency on carbamazepine compared to DBD plasma. This effect was recognized by the generation of N oxides, and the consequent reactions with OH radicals or/and O3, which decreased the oxidizer levels to prevent chain reactions instigated by O3 [84]. Similarly, Ibuprofen degradation in water was achieved in another study by pulsed corona discharge of NTAPP over liquid, produced in O2. Interestingly, complete Ibuprofen removal was achieved within 20 min of NTAPP exposure, and the mineralization level increased to 76% after 1 h. Another group proposed a plasma-ozonation combined system, where suitable mass transmission of the NTAPP-produced O3 to the treated liquid was confirmed [85]. The effect of the discharge pulse on the Ibuprofen elimination revealed that a considerable increase in efficiency could be attained by pulse size reduction [85].
Figure 7. Application of NTAPP for the degradation of pharmaceutical compounds in water (reprinted with permission from [68], Copyright Elsevier (2015)).
To extend these combination-based procedures for wastewater purification, application-directed pilot-scale analysis was performed by combining ultrafiltration and an NTAPP system. This generator system creates air in plasma, and a corresponding proposed turbine supplies the oxidizing species to the water that must be treated. This process has been applied for the management of sewage from a slurry wastewater treatment plant. Prior treatment with ultrafiltration eliminated a large portion of pharma drugs and additional oxidable constituents, eventually reducing the O3 supply and improving the removal efficiency. Treatment using ultrafiltration or NTAPP eliminated these pharmaceuticals pollutants to a greater degree, although the percentage removed did not exceed 90%. This study suggested that the use of NTAPP technology with ultrafiltration is a feasible and energy-efficient treatment approach for large-scale wastewater treatment [86]. Treatment approaches by using different NTAAP sources to remove the different chemical pollutants are summarized in Table 2.
Table 2. Treatment approaches used for different pollutants.
| Plasma Source | Chemical Pollutants | Refs. |
|---|---|---|
| DBD reactor | Bisphenol A and tributyltin | [58] |
| DBD device | Organophosphate pesticide(dimethoate and dichlorvos) | [59] |
| Glow discharge | Saline and azo dye (AR 73) | [60] |
| Combination of air stripping and NTAPP | M-xylene and toluene | [64] |
| Microplasma-based AOP process | DMP removal | [73] |
| DBD reactor | Verapamil oxidation | [81] |
| Low-power ex situ plasma treatment | carbamazepine | [84] |
| Pulsed corona discharge NTAPP | Ibuprofen degradation | [85] |
| Combination of ultrafiltration and an NTAPP system | Slurry wastewater treatment | [86] |