1000/1000
Hot
Most Recent

Since 2011, following the tragic Fukushima Daiichi Nuclear accident, great attention has been devoted to the development of a new concept of nuclear fuel to improve the safety of nuclear reactors during normal operation, transient modes and under accident conditions. A concept called “accident tolerant fuel (ATF)” indicates a strategy to prevent/limit the interaction of cladding material with water steam, or hydrogen embrittlement, and to reduce heat generation during cladding oxidation and increase “processing time” under accident conditions before re-flooding of the nuclear core.
In recent decades, zirconium-based alloys have been used in the nuclear industry as the main material for fuel claddings and other structural components of pressurized and boiling water reactors (PWRs and BWRs), Russian water–water energetic reactors (VVERs) and high-power channel-type reactors (RBMK) [1]. During normal operation, zirconium alloys form a protective zirconium oxide layer that reduces cladding corrosion in the coolant. The key factors affecting claddings degradation are radiation swelling and embrittlement caused by oxidation and hydrogenation of the zirconium claddings [2][3][4]. However, zirconium alloys demonstrate poor oxidation kinetics at elevated temperatures.
The most crucial conditions for fuel claddings can occur in the case of a loss of coolant accident (LOCA) [1][5]. LOCA events can be caused by a breakup of the primary cooling system that results in the loss of pressure in the nuclear core and vaporization of the coolant. Under these conditions, the fuel temperature rises, thereby increasing the porosity of the fuel and resulting in its fragmentation. The fuel cladding temperature also increases abruptly. When Zr-based (E110, E635, Zircaloys, M5, ZIRLO, etc.) claddings interact with water steam at a high temperature (above 800 °C), it causes oxidation and embrittlement by releasing additional heat due to the exothermic reaction:
Therefore, the development of ATF cladding material[6][7][8] is desperately needed to enhance the robustness of LWRs in normal and possible accident conditions.
Nowadays, two main ATF strategies are considered. The first is to replace current cladding material with SiCf/SiC composites [9][10][11][12], FeCrAl [13][14][15][16][17][18][19][20][21][22][23][24][25][26][27][28][29][30][31][32][33][34][35][36][37][38][39][40][41][42][43][44][45][46][47][48][49][50][51][52][53][54][55][56][57][58][59][60][61][62][63][64][65][66][67][68][69][70][71][72][73][74][75][76][77][78][79][80][81][82][83][84][85][86][87][88][89][90][91][92][93][94][95][96][97][98][99][100][101][102][103][104][105][106][107][108][109][110][111][112][113][114][115][116][117], molybdenum alloys [16][18][19][20][21] or Ni-based stainless steels [22]. Taking into account the duration and possible total costs for developing a new type of cladding material, this way is considered as a long-term strategy. The second ATF concept is the development of protective coatings on the surface of Zr fuel claddings [23][24]. Protective coatings should strongly improve corrosion and high-temperature (HT) oxidation resistance, wear-resistance and reduce hydrogen absorption of Zr-based alloys used in LWRs. Therefore, coating technology can be added to the technological process of nuclear fuel production that can be achieved in a short-term period. Despite the simplicity of this approach, a large number of possible factors (coating adhesion, thermal conductivity, thermal neutron cross-section, radiation resistance, mechanical properties [25]) can affect the behavior of coated Zr claddings in both normal and accident conditions. Currently, numerous studies are being performed to improve zirconium alloy performance by the deposition of metallic (Fe-based alloys, Cr, Cr-Al, Y, Ni-Cr, etc.), non-metallic (oxides, nitrides, carbides) or MAX-phase coatings [15][26][27][28]. Among a variety of coatings, the highest performance in LOCA scenarios belongs to materials which can produce oxide phases such as alumina, zirconia, chromia or silica [29][30][31][32].
Numerous studies have been performed to improve zirconium alloys’ performance under both normal and accident conditions by the deposition of protective coatings [60][61][62][63]. Coated Zr alloys show enhanced corrosion and HT oxidation resistance; although reduced, relatively thin coatings are not influenced by the thermomechanical behavior of Zr-based claddings [69], the coatings should not noticeably change neutron absorption in LWRs [70] and heat transfer between the cladding and coolant [71].
Currently, there are two major research institutes focusing on the development of FeCrAl coatings for nuclear fuel claddings. These are the Korea Atomic Energy Research Institute (KAERI) and the University of Illinois Urbana-Champaign (UIUC). Fe-based coatings on Zr necessitate a barrier layer between the coating and substrate to mitigate Zr-Fe eutectic formation at high temperatures [76]. Zhong et al. investigated the oxidation performance of FeCrAl coatings under HT steam conditions [77]. In this study, FeCrAl was deposited on Zry-2 alloy using magnetron sputtering. Based on the experimental results, 1 µm-thick FeCrAl coating prevented the oxidation of the Zry-2 substrate in steam at 700 °C up to 15 h, while a thin FeCrAl (0.3 µm) film did not protect the alloy. Furthermore, investigations performed under autoclave testing (20 days) in a simulated BWR environment demonstrated an adequate performance without the loss of coating integrity. Kim et al. investigated the surface-modified Zr cladding concept: an outer FeCrAl protective layer was chosen as a coating to enhance the oxidation/corrosion resistance of Zr alloy deposited by laser coating and arc ion implantation methods to increase the adhesion strength of the coating and the Zr alloy tubes [78]. The obtained samples demonstrate improved corrosion/oxidation performance, creep and irradiation resistance compared to commercial Zr claddings. Good radiation resistance was also observed for FeCrAl alloys under heavy ion irradiation [79]. There was no void formation or Cr-enriched phases in 10Cr and 13Cr FeCrAl alloys, and irradiation-induced defects only contributed to hardening. Dabney et al. showed that thick FeCrAl coatings obtained by cold spraying demonstrate high oxidation resistance during autoclave tests and HT oxidation in the air [80]. However, a fast diffusion of Fe from FeCrAl coating to Zr alloys caused a thick interlayer composed of (Fe,Cr)2Zr, FeZr3 and FeZr2 Laves phases (Figure 1a,b) [81].
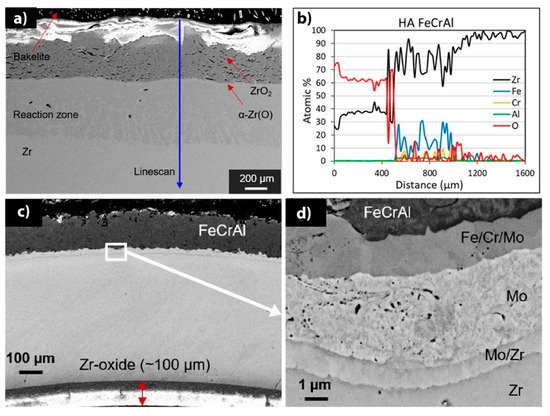
Figure 1. Cross-section SEM images after 1200 °C air oxidation: (a) FeCrAl-coated Zircaloy-4 and (b) corresponding EDS line scan; (c) FeCrAl/Mo coating and (d) magnified image of the FeCrAl/Mo/Zr interface. Reproduced from [80] with permission by Elsevier.
Park et al. investigated FeCrAl coatings deposited onto Zr substrates using cold spraying techniques [82]. For the FeCrAl/Zr system, a Mo layer was deposited between the FeCrAl coating and the Zr substrate to inhibit interdiffusion at high temperatures. As a result of mutual diffusion in the FeCrAl/Mo/Zr system, a complex multilayer structure was formed (Figure 1c,d). The FeCrAl coatings improved the oxidation resistance of the Zr alloy when exposed to a steam environment at 1200 °C. The ballooning behavior and mechanical properties of the coated samples studied under simulated LOCA conditions revealed higher burst temperatures, lower circumferential strain and smaller ruptures compared to the bare Zr. In addition, four-point bending and ring compression tests indicated a minimal increase in the maximum load and higher residual ductility of the coated Zr alloy, respectively [82].
Metallic Cr-based coatings are most promising in that they meet the basic requirements for ATF coating materials for Zr fuel claddings. Chromium has a high melting point, high corrosion resistance in water and steam due to a protective chromia scale, and a coefficient of thermal expansion similar to zirconium alloys. A wide variety of methods were implemented to deposit Cr coatings on Zr alloys such as magnetron sputtering [87], cathodic arc deposition [88], cold spraying [89], 3D laser cladding [90], electroplating [91], etc. The high potential of these coatings is also confirmed by the involvement of large industrial/research institutes such as CEA (France), VNIINM (Russia), KIT (Germany) and others. Several full-scale tests with Cr-coated Zr claddings with UO2 fuel in nuclear reactors are being performed at present.
Good protective properties of Cr coatings were demonstrated under normal operation conditions: autoclave testing in PWR and BWR simulated medium [61][62][63][88]. Wei et al. showed high corrosion resistance in Cr-coated Zry-4 alloy in both H3BO3-LiOH and dissolved oxygen containing water [63], showing only 50–100 nm stable Cr2O3 scale after a 3000 h autoclave test. Chromium coatings deposited on Zry-4 alloy exhibited promising performance under steady-state, power ramp and LOCA transient conditions [89][90]. Brachet et al. showed good protective properties of a 5–12 µm thick Cr coating under steam oxidation at 1200 °C (Figure 2) [83]. It was demonstrated that the coating enhanced the oxidation resistance of the alloy and ensured the integrity of the cladding for a longer oxidation time (approx. 10 times longer than current LOCA criteria). Ma et al. showed that Cr coatings could protect Zr-1Nb alloy from HT steam oxidation at 1200 and 1300 °C (Figure 3) [91]. It was shown that the thickness of the formed Cr2O3 and residual Cr layers depends on oxidation time, which is probably due to the combined effect of the ongoing redox reactions between chromium oxide and zirconium, also observed by Han et al. [92].
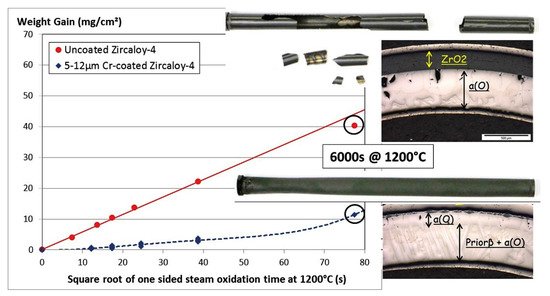
Figure 2. Optical images and weight gain evolution of uncoated and Cr-coated (5–12 μm) Zry-4 alloy during steam oxidation at 1200 °C. Reproduced from [83] with permission by Elsevier.
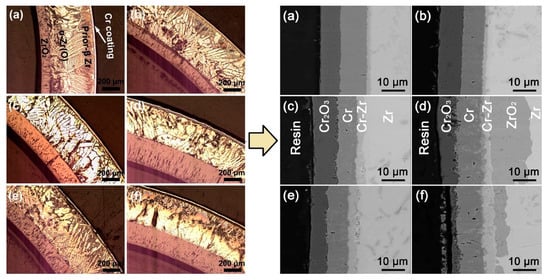
Figure 3. Optical and SEM images of Cr-coated zirconium alloy cladding after high-temperature steam oxidation at 1200 °C for 0.5 h (a), 1 h (b), 2 h (c), 4 h (d), and 1300 °C for 0.5 h (e) and 1 h (f). Reproduced from [91].
Results of mechanical tests under ambient and elevated (400 °C) temperatures showed no significant difference between the Cr-coated and the uncoated Zr claddings [61][86][93]. Kim et al. investigated the mechanical behavior of Zry-4 alloy with 3D laser-coated Cr coatings (80–120 µm) under ring compression and tensile tests [86]. The results indicate good adhesion and no defects in the Cr coatings up to 4% strain. The generation of cracks occurred at 6% strain without coating spallation or peeling. Ribis et al. analyzed the atomic-scale structure of the Cr/Zry-4 interface where the Cr coating was deposited using PVD method [94]. It was found that a thin layer of Laves Zr(Cr,Fe)2 phase with C14 and C15 polytypes was formed at the interface, while the coherent boundaries ensured good adhesion of the coating.
The adhesion behavior of Cr coatings was also affected by oxidation and diffusion processes at the coating/alloy interface [68]. Jiang et al. performed a comparative analysis of cracking resistance of multi-arc ion plated Cr and CrN coatings on Zry-4 alloy under tensile loading [95]. It was revealed that Cr coatings exhibit a brittle-to-ductile transition under thermomechanical loading, which results in their better cracking resistance compared to CrN coatings.
Several studies presented a good radiation resistance of chromium coatings under ion irradiation [96][97][98]. The stabilization of C14 and disappearance of C15 polytypes in the Cr/Zry-4 samples due to continuous incoming Fe flux were found during 20 MeV Kr+ irradiation at 400 °C. Despite this, the samples retained their adhesion and microstructure stability after ion irradiation [96]. An acceptable swelling of ~1.6% was observed at an irradiation temperature of 500 °C under 1.4 MeV heavy ion radiation up to 25 dpa [97]. This is twice lower than the allowable swelling value (~5%) for reactor materials.
Cr-Al alloy coatings show better oxidation resistance than pure chromium [76][99][100]. However, a more complex and heterogeneous structure of the oxide layers forms after oxidation [101], which requires more detailed studies under long-term HT steam oxidation, as well as B-DBA conditions. Ni-Cr coatings exhibit better ductility than pure Cr coatings but, at high temperatures, they show lower oxidation resistance and rapid diffusion of Ni inside the zirconium alloy occurs [102][103].
The resistance of yttrium to oxidation, as well as the formation of stable protective yttrium oxides at very high temperatures, made it very attractive for study. Sridharan et al. investigated surface modified Y-coated Zry-2 alloy and observed a decrease in the oxidation rate in supercritical water at 400 °C for 7 days (168 h) [104]. Kim et al. investigated the surface modification of 2 mm-thick Zry-4 alloy by deposition of Y2O3 (10 µm) using a laser beam scan method to produce an oxide dispersion strengthening (ODS) treatment [105]. It has been shown that the yield strength of Zry-4 with the ODS layer was 65% higher compared to uncoated alloy at 500 °C, therefore enhancing high-temperature strength to defeat the ballooning behavior of fuel cladding during an accident event [106]. However, the limited research on these types of coatings does not allow us to confirm the feasibility of their use as ATF materials.
High entropy alloys (HEA), also called multi-principal component alloys, are solid solutions of at least four elements in near equimolar ratios [107][108]. HEAs demonstrate a wide range of outstanding properties, such as high thermal stability, corrosion resistance and radiation damage tolerance [109][110]. Zhang et al. investigated corrosion behavior of a 3 µm-thick AlCrMoNbZr HEA coating deposited on an N36 alloy (Zr-1Sn-1Nb-0.3Fe) in static water at 360 °C and 18.7 MPa for 30 days [111]. It was reported that the oxidation resistance of the N36 alloy increased threefold due to multiphase oxide phases (Nb2Zr6O17, ZrO2 and Cr2O3) formed at the surface. A multilayer AlCrMoNbZr/(AlCrMoNbZr)N coating can be beneficial for preventing Al migration and boehmite phase formation during autoclave corrosion of an AlCrMoNbZr alloy [112]. The multilayer AlCrMoNbZr/(AlCrMoNbZr)N coating with the layer step of 50 nm demonstrated better protective properties compared to 5/5 nm and 10/10 nm multilayers and single-layer AlCrMoNbZr (Figure 4) [113]. Despite the potential application of HEAs as ATF materials for fuel claddings [114][115][116][117], their application as protective coatings is challenging due to possible low temperature eutectics, with Zr alloys and complex oxide scales formed after HT oxidation.
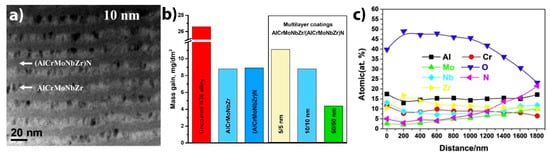
Figure 6. Multilayer AlCrMoNbZr/(AlCrMoNbZr)N HEA coatings: (a) cross section TEM image of 10/10 nm coating; (b) mass gains for HEA-coated N36 zirconium alloy after an autoclave test at 360 °C, 18.7 MPa for 30 days; (c) depth distribution of elements in 50/50 nm HEA coating after the autoclave test. Reproduced from [113] with permission by Elsevier.
There are several research groups working on the development of nitride coatings for nuclear fuel Zr claddings. Khatkhatay et al. deposited TiN and Ti0.35Al0.65N coatings on Zry-4 substrates using pulsed laser deposition, and exposed them to supercritical water conditions for 48 h at a temperature of 500 °C and a pressure of 25 MPa [118]. The coated tubes were remarkably intact after exposure, while uncoated tubes demonstrated severe oxidation and breakaway corrosion. The TiN coatings also reduce the hydrogenation of Zr alloys [119]. However, nanocrystalline TiN coatings can dissociate under energetic particle bombardment, forming Ti-enriched zones with low oxidation resistance [120]. Despite good diffusion barrier properties of TiN coatings [121], they are considered less as a protective coating or barrier interlayer because of their large coefficient of thermal expansion (CTE) difference with Zr and possible cracking during thermal cycling [122]. Alat et al. studied the corrosion resistance of TiAlN and TiN coatings deposited on ZIRLO substrates by cathodic arc physical vapor deposition [123][124]. The corrosion tests were implemented in static pure water at 360 °C and 18.7 MPa for 72 h. After the tests, a very low weight gain between 1–5 mg/dm2 was observed, whereas the uncoated ZIRLO samples showed an average weight gain of 14.4 mg/dm2. However, aluminum depletion in a high-temperature water environment results in the formation of a boehmite phase that degrades the corrosion resistance of TiAlN coatings (Figure 5). To eliminate boehmite formation, the deposition of an outer TiN layer or multilayer approach based on TiN/TiAlN coating can be effective [124][125].
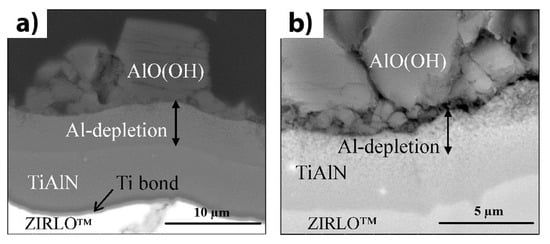
Figure 5. Cross-section SEM images of TiAlN/Ti/ZIRLO™ after autoclave testing at 360 °C for 72 h: (a) secondary electron mode, and (b) backscattered electron mode. Reproduced from [123] with permission by Elsevier.
Daub et al. provided comparative analyses on corrosion resistance of 2–4 µm-thick CrN-, TiAlN- and AlCrN-coated Zry-4 alloy [126][127]. It was shown that a CrN coating demonstrates better overall performance in both aqueous and steam environments, as well as twice-reduced hydrogen ingress. Meng et al. showed the high resistance of a 13 µm-thick CrN coating deposited on a Zr-702 alloy in the air up to 1160 °C (Figure 6) [128]. Krejčí et al. demonstrated cracking and local failures of CrN coatings after HT steam oxidation at 1200 °C for 30 min [129]. It was indicated that the cracking was caused by partial decomposition of CrN to Cr2N at high temperatures (typically below 850 °C) [130].
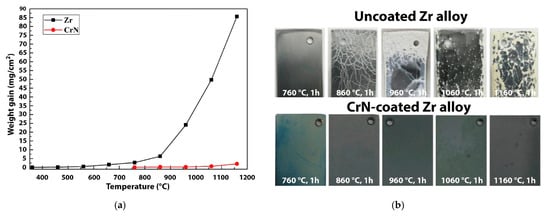
Figure 6. Weight gain of uncoated and CrN-coated Zr alloys as the function of oxidation temperature in the air (a), and the appearance of the samples after the oxidation tests (b). Reproduced from [128] with permission by Elsevier.
Zirconium silicides have high thermal conductivity and favorable mechanical properties [131]. Yeom et al. studied the oxidation behavior of Zr2Si, ZrSi and ZrSi2 coatings deposited on a Zry-4 alloy by magnetron sputtering [132]. Thicker (3.9 µm) ZrSi2 coating exhibited oxidation resistance almost two orders of magnitude higher compared to uncoated Zry-4 in 700 °C air for 20 h. The thicknesses of the oxide layers were 7 and 20 μm for coated Zry-4 at 1000 (1 h) and 1200 °C (10 min) steam, respectively. No cracking or spallation was observed after three cycles of water quenching from 700 °C. However, the brittle nature of silicide coatings and coating volatilization under autoclave testing limit their application for ATF [133].
Silicon carbide is a promising material for ATF Zr claddings since it has a high melting point, low chemical reactivity, superior oxidation resistance at high temperatures and a lower thermal neutron cross-section than Zr-based alloys [134]. SiC coatings deposited on a Zry-4 alloy using co-sputtering of SiC and Si targets demonstrate enhanced oxidation resistance in 900 °C steam due to the formation of a protective silica layer [135]. SiC coatings obtained by magnetron sputtering also reduce the hydrogenation of zirconium alloys under normal operation temperatures [136][137]. The effect of deposition parameters on the mechanical properties of SiC coatings deposited on Zircaloy-4 substrate by magnetron sputtering was shown in [138]. Al-Olayyan et al. showed better adhesion of SiC coating on rough surfaces of Zry-4 alloy, which resulted in higher corrosion resistance [139]. Nevertheless, unstable oxide growth and coating volatilization during autoclave corrosion tests must be considered carefully when designing SiC-based coatings for LWRs [140].
Zr-Al-C coatings obtained on Zry-4 alloy by magnetron sputtering demonstrated poor protective properties during oxidation in steam at temperatures beyond 800 °C due to formation of ZrO2 and Al2O3 oxides scales [141]. Michau et al. studied the protective properties of Cr-based carbide (CrC, CrSiC) coatings deposited on the inner surface of Zr cladding tubes using DLI-MOCVD [142]. Good adhesion and better oxidation resistance of amorphous CrxCy coating in air and steam at 1200 °C were shown (Figure 7), while the addition of Si was ineffective in improving oxidation resistance of chromium carbide coatings. It was also shown that an optimized DLI-MOCVD process can be successfully used to deposit CrCx coatings inside Zr cladding tubes 1 m in length [143][144].
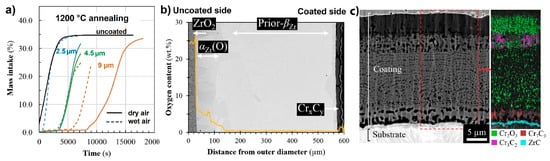
Figure 7. Mass gains measured by TGA of uncoated and CrxCy-coated Zircaloy-4 under air oxidation isothermal air oxidation at 1200 °C (a); BSE SEM image and oxygen profiles of CrxCy-coated clad segment after oxidation in steam at 1200 °C for 10 min, followed by water quenching (b); and the detailed SEM + EBSD image of the inner surface of CrxCy-coated clad segment after the oxidation test (c). Reproduced from [142] with permission by Elsevier.
Jin et al. deposited 75Cr3C2-25NiCr (wt.%) on a Zr-2.5Nb alloy using a high velocity oxygen fuel (HVOF) technique and showed improved oxidation resistance in air and steam at 700–1000 °C [103]. However, the coated samples showed poor corrosion resistance in supercritical water at 400 °C and 10.3 MPa, caused by a bimetallic effect (this coating can build up bimetallic couple with the Zr-2.5Nb alloy, resulting in the acceleration of the corrosion rate). Yang et al. demonstrated high oxidation resistance of 120 μm-thick HVOF Cr3C2-NiCr coating at 1200 °C in steam for 1 h due to the formation of a dense chromia scale [145]. However, the high coating thickness and heterogeneous/porous microstructure formed by the HVOF method may limit the usefulness of such coatings for ATF Zr claddings.
MAX phases are layered hexagonal carbides and nitrides referenced to the general formula Mn+1AXn, (MAX), where “n” ranges between 1 and 4, “M” represents a transition metal (Sc, Ti, V, Cr, etc.), “A” is an A-group (mainly IIIA and IVA, or groups 13 and 14) element and “X” is either C and/or N [146][147]. MAX phase materials exhibit excellent properties of both metals and ceramics such as high electrical and thermal conductivity, high melting points and excellent oxidation resistance [148][149].
Alumina-forming MAX phases demonstrate superior oxidation resistance at high temperatures and exhibit self-healing ability [150]. Li et al. investigated high-temperature oxidation resistance of 12 μm-thick Ti2AlC coating on ZIRLO alloy in pure steam at 1000–1200 °C [151]. The coating remains intact and demonstrates good protective properties up to 1200 °C during 5 min oxidation time. The improved oxidation resistance was due to the dense columnar-free microstructure of Ti2AlC coating and triple oxide scale α-Al2O3 + rutile-TiO2/α-Al2O3/TiO2. Maier et al. showed the oxidation resistance of cold sprayed Ti2AlC coating (90 μm) in Ar/steam mixture at 1005 °C for 20 min [152]. The Ti2AlC coating protected the Zry-4 alloy from oxidation and had high hardness and wear resistance. Thinner Ti2AlC/TiC coatings (5/0.5 μm) deposited by magnetron sputtering demonstrate high oxidation resistance in steam at 800 °C, forming a triple-layered scale (θ-Al2O3 + TiO2/θ-Al2O3/TiO2) [153]. It was also shown that the TiC barrier layer mitigates the inward diffusion of Al into the Zry-4 alloy. However, rapid oxidation at 1000 °C resulted in cracking and spallation of the Ti2AlC coatings.
The oxidation resistance of Ti-Al-C and Cr-Al-C coatings deposited by magnetron sputtering on ZIRLO alloy was investigated in [154]. Results of the autoclave test at 360 °C and 18.6 MPa showed poor protective properties of Ti-Al-C coatings due to the formation of multiple oxide scale and hydroxide phases. The Cr2AlC coating demonstrates better oxidation performance; however, partial spallation and formation of volatilized AlOOH was also revealed. Tang et al. showed the excellent oxidation resistance and self-healing ability of thin Cr2AlC coatings in steam at 1000 °C (Figure 8) [155].

Figure 8. Cross-section images of three types of MAX-phase coatings with barrier layers on Zircaloy-4 after annealing: (a) Ti2AlC, (b) Zr(Al)C, (c) Cr2AlC, and their oxidation kinetics in 1000 °C steam (d). Reproduced from [155] with permission from the authors.
Imtyazuddin et al. analyzed the radiation resistance of magnetron sputtered Cr2AlC films under 320 keV Xe ions at 300 and 623 K [156]. It was found that Cr2AlC films are amorphized at room temperature even at low doses; however, no amorphous phase was found up to 90 dpa at 623 K, indicating good radiation tolerance at elevated temperatures (Figure 9). Furthermore, both Ti2AlC and Cr2AlC coatings reduce hydrogen uptake effectively [157]. Wang et al. also demonstrated good oxidation resistance of 10 μm-thick Cr2AlC coatings in air at 1100 °C [158].
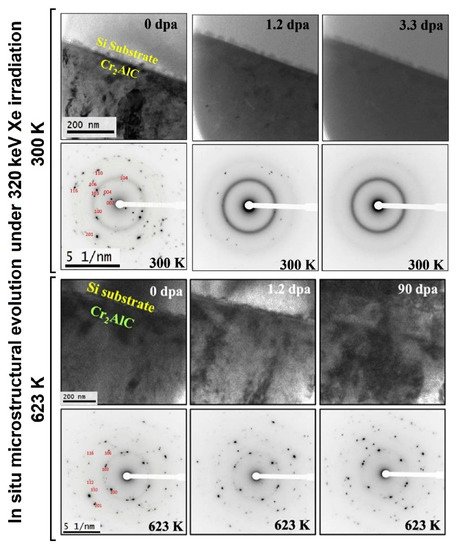
Figure 9. Evolution of microstructure of magnetron-deposited Cr2AlC thin film under 320 keV Xe irradiation at 300 and 623 K. Reproduced from [156] with permission by Elsevier.
A literature review indicates the rapid diffusion of “A” element from the MAX phases into the Zr alloys with the formation of an interdiffusion layer, thereby decreasing the protective properties of the coating. Thus, various diffusion barrier layers between the coating and the substrate should be considered when developing protective coatings for ATF based on MAX-phases.