1000/1000
Hot
Most Recent

Hydrocephalus is a common complication of aneurysmal subarachnoid hemorrhage (aSAH) and reportedly contributes to poor neurological outcomes.
Aneurysmal subarachnoid hemorrhage (aSAH) remains a devastating disease that is characterized by a high mortality rate and significant morbidity amongst survivors [1][2]. Hydrocephalus is a frequently encountered complication following aSAH and is classified as acute (0–3 days post-SAH), subacute (4–13 days post-SAH), or chronic (14 days post-SAH) [3][4]. Acute hydrocephalus necessitates the placement of an external ventricular drain (EVD) to reduce deleterious secondary effects after the aneurysm bleed, with up to 48% of EVD recipients requiring ventriculoperitoneal (VP) shunt insertion [5][6][7].
Various mechanisms have been implicated as causative factors for the development of chronic hydrocephalus following aSAH, including alterations in CSF dynamics, obstruction of the arachnoid granulations by blood products, and adhesions within the ventricular system [8][9][10].
Chronic hydrocephalus following aSAH reportedly contributes to poor neurological outcomes and severe cognitive deficits [11][12][13][14] (Figure 1). The development of predictive models that could stratify patients with aSAH based on their risk of developing shunt-dependent chronic hydrocephalus is important.
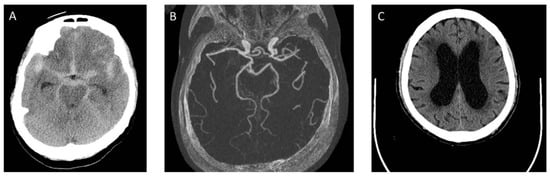
Figure 1. The series of computed tomography (CT) images of a case with aSAH. (A) A 68 years-old female patient presented with SAH at admission. There is no IVH and no acute hydrocephalus; (B) a ruptured left posterior communicating artery aneurysm was identified and coiled; (C) on 12 days after aSAH, CT showed hydrocephalus. She developed progressive ataxia and cognitive dysfunction.
Following aSAH, evidence suggests that extensive fibrosis in the subarachnoid space may be an important cause of chronic hydrocephalus development [8][15].
TGF-β1 levels have been reported to be higher in the CSF following aSAH, especially in patients with hydrocephalus, which implies its role in the pathogenesis of subarachnoid fibrosis and chronic hydrocephalus following aSAH [16][17][18]. Some TGF-β1 antagonists and TGF-β1 signaling pathway inhibitors alleviate chronic hydrocephalus and improve behavioral outcomes in rat aSAH models [19][20].
TNC may cause leptomeningeal collagen synthesis and fibrosis, as well as brain injuries with decreased brain parenchymal volume contributing to subsequent ventricular enlargement, resulting in the development of chronic hydrocephalus, which is consistent with findings from a CSF study in humans [21][22].
SAH causes a systemic inflammatory response syndrome (SIRS) that involves complex interactions amongst immune cells, inflammation, coagulation, sympathoadrenal activation, endothelial cell activation, and dysfunction [23]. This complex process leads to a procoagulant reaction, tissue hypoperfusion, microthrombosis, and compromised microcirculation, which ultimately leads to multiorgan failure [23][24][25]. Release of catecholamines into the systemic circulation following aSAH may cause arrhythmias and neurogenic pulmonary edema.
The immune response following aSAH has been described in recent publications. Following aSAH, systemic IL-6 levels increase rapidly, whereas IL-10 levels are reduced [26]. Neutrophils are increased both in the brain and in the blood, reflecting local and peripheral inflammation following aSAH [27]. Higher levels of intracerebral proinflammatory monocytes are found within 24 h than after 1 week [26]. In addition to stroke-related SIRS, immune dysregulation plays an important role in brain injury and recovery. For example, the spleen contracts following ischemic stroke, activating a peripheral immune response that may exacerbate ongoing brain injury [28].
The rate of shunt dependency after treated aSAH ranges from 17.2% to 31.2% [14][29]. The four mechanisms underlying the pathophysiology of aSAH-induced brain injury are acute obstructive hydrocephalus, a mass effect exerted by IVH, SAH-related cytotoxic blood degradation products in the adjacent brain tissue, and chronic hydrocephalus [30] (Figure 2). Acute hydrocephalus requires EVD management, while using EVD may also cause chronic hydrocephalus. When chronic hydrocephalus progresses, EVD cannot be removed and may need a permanent shunt. The clearance of blood clots from the ventricles has therefore become a major therapeutic goal.
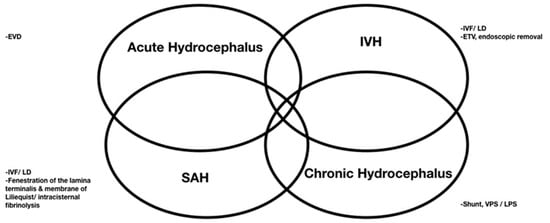
Figure 2. Surgeries applied for four mechanisms underlying the pathophysiology of aSAH-induced brain injury.
Endoscopic IVH removal can be useful in certain cases. Although the published series on endoscopic IVH removal detail results similar to those with IVF, no randomized trial has proven the superiority of this approach over EVD + IVF or EVD alone. Although the combination of coiling and endoscopic IVH removal in aSAH patients has proven safe and effective, given the technical demand of endoscopic IVH removal, evidence demonstrating its superiority over EVD + IVF is needed before it can be widely adopted [31][32][33][34]. This can be performed in the hybrid room with a multidisciplinary team with coiling performed before or after endoscopic IVH removal [33][34]. The hybrid operating room enables the two treatment approaches to be performed without the need to transfer the patient, and thereby minimizes the transition time between the modalities.
Although the influence of treatment modality (surgical clipping versus endovascular coiling) on shunt dependency remains controversial, it has been postulated that open surgery allows irrigation and removal of subarachnoid clots and thereby reduces the probability of chronic hydrocephalus [35][36]. Some studies have suggested that endovascular treatment is independently associated with the development of chronic hydrocephalus in aSAH patients [36][37]. However, other research has demonstrated a significantly lower incidence of chronic hydrocephalus after endovascular treatment compared with after surgical clipping [38].
Some surgical techniques have been shown to decrease the risk of shunt dependency. Tandem fenestration of the lamina terminalis and membrane of Liliequist has been shown to decrease shunt dependency following surgical clipping or bypass following aSAH (17.9% vs. 3.2%) [39].
To sum up, the development of shunt-dependent hydrocephalus following aSAH is multifactorial. The involvement of multiple cellular signaling pathways and inflammatory responses all contribute to its pathogenesis. This review integrates the updated knowledge about the clinical, molecular, prognostic, and therapeutic aspects of chronic hydrocephalus following aSAH. Recent literature has shown that prolonged use of EVD in the acute stage may lead to subsequent hydrocephalus. There is a need to standardize the EVD weaning process to decrease shunt dependency. Although lumbar drainage and IVF may decrease shunt dependency, those still need further validation. There have been no ideal treatment approaches for this devastating disease which points to a need for therapeutic strategies to target these underlying mechanisms. Understanding the risk factors and molecular pathophysiology related to the development of hydrocephalus following aSAH may help neurosurgeons to determine the intervention targeting these factors.