1000/1000
Hot
Most Recent

Mas-related G-protein coupled receptor member X2 (MRGPRX2) is a class A GPCR expressed on mast cells. Mast cells are granulated tissue-resident cells known for host cell response, allergic response, and vascular homeostasis.
The pseudo-allergic reaction is a hypersensitivity reaction manifested by immediate systemic responses. The symptoms are identical to anaphylaxis; however, pseudo-allergic reaction shares a different mechanism of mast cells (MCs) activation to that of anaphylaxis (Immunoglobulin E (IgE)-mediated) [1]. Pseudo-allergic reactions are non-IgE-mediated hypersensitivity reactions which are elicited by an initial dose of medication, and cause MC degranulation followed by the release of inflammatory and pro-inflammatory mediators [2]. It is worth noting that these reactions do not elicit antigen-specific immune responses but evoke the release of histamine and cytokines, activate the complement system, and lead to atypical synthesis of eicosanoids [3][4]. Previously, hypersensitivity reactions (HSRs) have been classified into four types; however, pseudo-allergic reactions are not categorized into any of these four classes [5][6]. One possibility is indistinguishable clinical signs and symptoms from anaphylaxis (IgE-mediated) [7][8] due to a lack of clear understanding of non-IgE-mediated mechanisms. The signs and symptoms of pseudo-allergic reactions are similar to IgE-mediated symptoms such as skin flushing, headache, edema, hypotension, urticaria, and bronchospasm [7][8]. Pseudo-allergic reactions attributed to two-thirds of HSRs [9]. However, the lack of systematic studies on the causes and mechanisms is the bottleneck for diagnosing and treating these reactions.
MRGPRX2 is a class A GPCR which binds with several endogenous and exogenous ligands. Previously, research on both MC degranulation and host response mechanism was focused on the IgE receptors (FcεRI)-mediated immune response. Investigating the alternative non-IgE mechanisms of MC degranulation and immune host response has gained immense interest recently. In the past 10 years, MRGPRX2 has emerged as a significant MC receptor responsible for non-IgE-mediated pseudo-allergic reactions. Accumulating evidence on MRGPRX2 has shown its critical role in drug-induced pseudo-allergic reactions, hypersensitivity reactions, and inflammatory diseases. MRGPRX2 is evolving as a versatile receptor with its diversity of ligands. More than 20 ligands (agonists or antagonists) have already been identified, and the quest for more agonists/antagonists that may solve the puzzle of non-IgE-mediated pseudo-allergic reactions is in full swing. However, the crucial pathophysiological roles of MRGPRX2 in allergic and non-allergic diseases remain poorly understood.
MCs are granulated immune cells that possess secretory granules which release several inflammatory mediators such as proteases and inflammatory cytokines [10]. Among these mediators, proteases are lineage-defining characteristics and are confined to MCs [11]. MC chymase and tryptase have been reported to expressed ≈10,000-fold higher than non-MCs, which makes them the most selective features of the lineage [12][13][14].
MCs are classified into two types based on the protease content of their secretory granules [15]. The first type is called MCTC, or tryptase and chymase-expressing MCs; this type is found in connective tissues, mainly in the skin, and other tissues such as intestinal submucosa and myocardium. The second type called MCT or tryptase-expressing MCs found mainly in the lung and gut [16][17]. Interestingly, in mice, the difference between human MCTC and MCT has been correlated, where connective tissue MCs (CTMCs) resemble MCTC, and mucosal MCs (MMCs) resemble MCT [18][19]. The mice connective tissue MCs are mainly found in the skin, and mucosal MCs are located in the lung and gut. Besides the difference in the protease content of MCs, several other features have been reported, distinguishing the MC subtypes. These features include ultrastructural differences, secretory functions, receptor expression differences, and pharmacological responses [20][21][22]. For instance, MCTC expresses carboxypeptidase A (CPA3), cathepsin G (CTSG) and complement component receptors (C5AR1 and C3AR1), while MCT does not express these mediators and receptors [23]. However, MCTC’s C5AR1 and C3AR1 receptors are less prominent than other immune cells such as monocytes, macrophages, neutrophils, eosinophils and basophils [13][14].
Moreover, there is a difference in the MC degranulation response in both subtypes of MCs against endogenous and exogenous stimuli. Human MC-subtype MCTC and mouse MC-subtype connective tissue MCs respond to ligands such as compound 48/80, complement components C3a, and C5a [20][21]. In contrast, the other subtypes of both humans and mice, MCT and mucosal MCs, respectively, do not respond to these ligands [24][25][26]. Such distinctive MC responses remained enigmatic for decades. In 2006, a novel MC receptor, MRGPRX2, was discovered [27], which is confined to MCTC, and is absent in MCT [12]. MRGPRX2 now serves as a novel signature marker of MCTC [27] and clearly explains the distinctive MC response against a plethora of agonists. MCTCs are abundantly present in the skin tissue [28] and are involved in both local and systemic immune responses such as skin allergy, anaphylaxis, and systemic mastocytosis [10][29]. The role of MCs in allergic diseases has been extensively studied, with recent findings also indicating the possible role of MCTC/connective tissue MC subtypes of MCs [19] in vascular hemostasis, pain, itch, and host defense [27][30][31][32].
MCs regulate the innate and adaptive immune responses via releasing stored or de novo-synthesized bioactive and inflammatory mediators [10]. During homeostasis, the immune response remains in tight regulation and control to avoid overshooting and self-induced host cell damage. The immediate hypersensitivity reactions are manifested due to the rapid release of inflammatory mediators from activated MCs. The canonical mast cell activation IgE pathway is a well-studied mechanism where the antigen binds with IgE and interacts with the Fc epsilon RI (FcεRI) high-affinity IgE receptor. FcεRI has been considered as the primary receptor on MCs for decades, responsible for MCs’ activated clinical manifestations, and is still a prioritized research focus in MC biology. Several other classes of MC receptors operate in a parallel fashion to induce the immune response, such as inflammation, while at the same time, these receptors ensure the resolution of the host response to xenobiotics and other inflammogens.
Moreover, the IgE MC activation mechanism is likely to be an over-simplification of real-time ongoing reactions inside the body. Several studies have reported that the immediate microenvironment surrounding the cells in their resident tissues and other receptors on MCs modify antigen-dependent MC activation [33]. Stem cell factor receptor (SCF/KIT) [34][35], TLRs [36], and several GPCRs [37] have been reported to modify MC responses elicited by aggregated FcεRI. These GPCR agonists elicit divergent pharmacological responses such as chemotaxis and adhesion to potentiation of FcεRI-mediated MC activation [33][37].
FcεRI is present on MCs’ surface and comprises three subunits, namely α, β and γ. The α subunit is the IgE binding site, the β subunit amplifies the signal, and two disulfide-linked γ subunits initiate the signals [38]. FcεRI-IgE aggregation elicits the tyrosine kinase cascade, culminating in granule exocytosis and rapid release of histamine and other proteolytic enzymes [37]. It is worth noting that IgE-mediated MC activation shows a relatively slow, enduring Ca2+ signal, compound exocytosis, and histamine is the major preformed mediator [39].
It is well known that only receptors that elicit degranulation can cause acute hypersensitivity reactions (HSRs) and allergic or pseudo-allergic reactions. Several GPCRs such as C5AR1, C3AR1, and recently identified MRGPRX2 can induce exocytosis, allergies, and anaphylactic responses. However, the clinical significance of complement receptors in HSRs has been extensively studied unlike the MRGPRX2, which is yet to explored [40][41]. Previously, the MCTC activation mechanism of several basic peptide molecules (such as substance P (SP)) and experimental drug compound 48/80 was not fully known. The MC activation mechanism of SP was reported through neurokinin 1 receptor (NK1R) [42] and direct activation of G proteins in the cytosol [43][44].
In 2006, for the first time, the selective expression and ligands activation mechanism of MRGPRX2 was reported [45]. GPCRs are membrane receptors expressed on plasma membrane; however, MRGPRX2 is also expressed in the intracellular sites of skin MCTC [46]. Ligands, such as SP, and compound 48/80 have shown MRGPRX2-dependent MC activity [45]. Although both NK1R and MRGPRX2 are expressed in MCTC, the SP-induced MC degranulation activity was dependent on MRGPRX2, not NK1R [46]. Additionally, the expression of MRGPRX2 is reported to be very high in MCTC (17,565 per 5 ng of total RNA) as compared to MCT (32 per 5 ng of total RNA) [45]. These findings are consistent with others where the PCR and microarray data demonstrated high transcription of MRGPRX2 in human skin MCTC, while MRGPRX2 transcription was low in lung MCT [46][47].
MCs produce and store several bioactive mediators that are released immediately or in a delayed fashion. The MC mediators show proinflammatory and immunomodulatory activity which causes other immune cells in the body to produce more inflammatory mediators. MCs contain several preformed mediators as well as de novo mediators. The preformed mediators are histamine, serotonin, β-hexosaminidase, interleukins, tryptase, chondroitin sulfate, and heparin. The de novo mediators are prostaglandins, cysteinyl leukotriene E4, platelet activating factor, and cytokines [10] (Figure 1). Several MRGPRX2 agonists such as human β-defensins, LL-37, and angiogenic peptide-30/5C have been reported to release histamine, interleukin 8, monocyte chemoattractant proteins, macrophage inflammatory protein, tumor necrosis factor-α, and prostaglandins [48][49][50]. The MCs’ released mediators manifest their effects via binding to several GPCRs such as histamine receptor 1–4 (histamine), protease activated receptor 2 (tryptase), cysteinyl leukotriene receptor 1–2 (LTC4), and prostaglandin D2 receptor (PGD2) on end-organ targets like epithelial cells, endothelial cells, airway smooth muscle cells and nerves [51]. The typical manifestation of MC-mediated allergic reactions is wheal, flare, and pruritus, due to histamine-mediated vasodilation and increased vascular permeability. Besides histamine, MC tryptases are a key mediator of histamine-independent itch through direct activation of protease-activated receptor 2 (PAR-2) on sensory nerves [52]. Tryptase has been reported to be involved in the pathophysiology of atopic dermatitis [53][54][55].
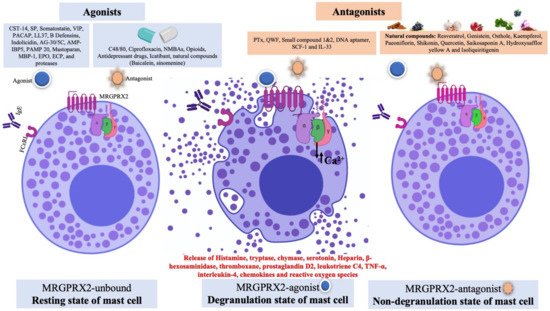
Figure 1. Schematic diagram showing mast cells receptors (FcεRI and MRGPRX2), MRGPRX2 ligands (agonist and antagonist) and different states of mast cells. The agonists are broadly divided into peptides and non-peptides (drugs). In an unbound state (where no ligand binds to MRGPRX2), the mast cells are in resting state. For agonists binding to MRGPRX2, the conformational changes in the receptor lead to activation of the G protein and downstream signaling pathway. This state is known as the degranulation state; the activated MRGPRX2 leads to exocytosis, MC degranulation and release of vasoactive and inflammatory mediators. However, the binding of antagonist to MRGPRX2 prevented receptor activation and inhibited MC degranulation and release of inflammatory mediators, which is known as the non-degranulation state.
The mechanisms of IgE- and MRGPRX2-mediated MC activation are different from each other. For example, in IgE-mediated MC activation, Ca2+ mobilization is relatively slow, which leads to compound exocytosis [56]. On the other hand, in MRGPRX2-mediated MC activation, Ca2+ mobilization is transient, which results in single granule fusion [56]. Moreover, there is a difference in the content of mediators release; histamine is the major preformed mediator in IgE, while tryptase is the major preformed mediator in MRGPRX2-mediated MC degranulation [57].
Thus far, mammals such as rodents, cattle, primates and, more recently, dogs have shown MRGPR-encoding genes [58][59]. The GPCR superfamily is classified into different classes, from A to F, according to sequence homology. Based on various conserved motifs, the MRGPR family is assigned to the rhodopsin-like class A GPCR [60][61][62]. The rhodopsin-like class A GPCR are further subdivided into four groups named A to D. MRGPR comes under the D group of class A and shares the same group as a large family of olfactory receptors, glycohormones, and purinergic receptors [60][61][62]. Still, no MRGPR subtype is declared deorphanized by NC-IUPHAR [63]. For instance, MRGPRX2, unlike any other GPCR, recognizes a wide variety of basic molecules and ligands; hence, there are numerous possible unknown ligands for this receptor. Therefore, it is still considered to be an orphan receptor regardless of possessing a plethora of ligands [64]. To deorphanize this receptor, at least two independent demonstrations of receptor–ligand pairing published in refereed papers are required [58]. Additionally, evidence of ligand binding in a given tissue via in vitro binding assays, functional assays, and anatomic data is crucial [58]. For MRGPR, at least one of the criteria mentioned is lacking; however, in a recent report in 2019 by IUPHAR/BPS, MRGPRX2 is listed as a class A orphan GPCR for which preliminary evidence for an endogenous ligand and potential disease link has been published [65].
Four MRGPRX members have been identified: MRGPRX1–X4, which shows different expression profiles and diverse functions. Briefly, MRGPRX1 is expressed mainly on small-diameter sensory dorsal root ganglia (DRG) nociceptive neurons and is involved in the perception of pain and itch sensations [66]. The human MRGPRX1 was the first primate-specific MRGPR to be assigned to a bovine adrenal medulla 8–22 (BAM8–22) ligand. After, several other agonistic and antagonistic molecules were identified [58][67].
The mouse genome possesses several family members of MRGPRs: MRGPRA (A1–A10), MRGPRB (B1–B5, B8), MRGPRC (C11), MRGPRD, MRGPRE, MRGPRF, MRGPRG, and MRGPRH genes [60][68]. MRGPRA1 and MRGPRB2 are considered as the mouse orthologs of human MRGPRX2 and share similar characteristics to MRGPRX2, such as exclusive expression on connective tissue MCs, DRGs and the ligand activation mechanism [69][70][71]. MRGPRA1 is expressed on DRGs and is activated by SP [60][71][72]. MRGPRB2 shows similar characteristics to MRGPRX2, such as exclusive expression on connective tissue MCs (not expressed on DRGs) and the ligand activation mechanism [60][71][72]. Immunochemistry studies have shown a high expression of MRGPRX2 in DRG neurons specifically, and the Cortistatin receptor is considered to be the most potent [73]. Additionally, MRGPRB2 is involved in neurogenic pain [74]; therefore, it can be speculated that MRGPRA1 is the major mouse ortholog that plays MRGPRX2’s role in DRG, whereas MRGPRB2 plays the role of MRGPRX2 in MCs.
MRGPRX2 is expressed on mast cells (MCTC but not in MCT subtypes) and dorsal root ganglions (DRGs) in humans and primates [45][73][75]. It is involved in host defense, pseudo-allergic reactions, non-histaminergic itch, periodontitis, neurogenic inflammation, and inflammatory pain [76][77][78]. MRGPRX2 binds with diverse agonists ranging from peptides to small molecules, insect venom chemical components, antimicrobial peptides, neuropeptides, and FDA-approved drugs [19]. MRGPRX3 and MRGPRX4 are expressed in human keratinocytes and are involved in cutaneous host defense response. There are not many studies reported on MRGPRX3–4 ligands; however, Toll-like receptor (TLR) ligands have been reported to upregulate the expression of MRGPRX3 and MRGPRX4 in human keratinocytes [79].
Although rodent MRGPRX2 (mouse MRGPRB2 and rat MRGPRB3) has similar characteristics such as selective expression in connective tissue MCs and similar ligand activation profiles [72], there is a considerable difference in efficacy of the agonists. For instance, the EC50 of several agonists for MRGPRB2 is higher than MRGPRX2 [72]. Additionally, the ligands’ selectivity in activation and inhibition of MRGPRB2/MRGPRX2 shows a considerable difference [71][80]. One possible reason can be the differences in the amino acid sequences of human MRGPRX2 and mouse MRGPRB2. Surprisingly, MRGPRX2/MRGPRB2 showed only ~53% overall sequence similarity, 34% N-terminal amino acids sequence similarity, and 47% C-terminal amino acids sequence similarity [19][45]. More recently, in 2020, dog MRGPRX2, which is the functional ortholog of human MRGPRX2, was identified [59]. Dog MRGPRX2 has shown selective expression in a limited number of skin tissues from the eyelid, abdomen, and cheek, which plays an essential role in drug-induced anaphylactoid reactions [59].
The human MRGPRX2 gene is a two-exon gene located on chromosome 11p15, 2036 bp in length, and encoding a protein with 330 amino acids [81][82][83]. The 3D structure of MRGPRX2 is yet to be identified; however, the homology modeling of the receptor revealed crucial structural information. The seven transmembrane (TM) bundles of MRGPRX2 are connected by three extracellular loops (ECL1, ECL2, and ECL3), and three intracellular loops (ICL1, ICL2, and ICL3). The N-terminus (N-term) of the extracellular (EC) domain is involved in ligand binding. The intracellular (IC) domain includes helix VIII and a C-terminal sequence is involved in G protein coupling and downstream signaling [84][85][86]. Recent experimental studies on MRGPRX2 structure biology have shown the crucial amino acids at the ECL and TM domain for binding and receptor activity [85][86][87][88] (Figure 2). The negatively charged residues, glutamic acid 164 (E164) and aspartic acid 184 (D184) at the ligand binding site of MRGPRX2, are crucial for binding several cationic ligands [69][87][88]. Since MRGPRX2 can be activated by a plethora of agonists, there might be more crucial amino acids that could be involved in ligand binding based on the nature of the ligand. In addition, there could be dual binding pockets for recognizing different ligands [69]. In TM domains, highly conserved class A GPCRs residues such as valine 123 (V123), isoleucine 225 (I225) and tyrosine 279 (Y279) are likely to participate in G protein coupling [86][89]. Furthermore, for receptor downstream signaling (G protein phosphorylation) at the carboxyl terminus of MRGPRX2, serine 313 (S313), threonine 321 (T321), serine 325 (S325), serine 327 (S327) and serine 328 (S328) are involved [86].
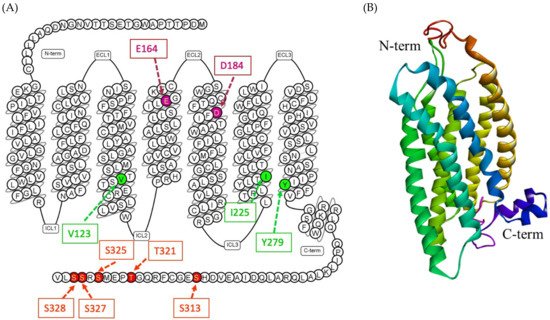
Figure 2. (A) Snake diagram of secondary structure of MRGPRX2 showing N-term, C-term, ECLs and ICLs, retrieved from GPCRdb (https://www.gpcrdb.org/, accessed on 15 April 2021) [90]. The amino acids involved in predicting the binding site of MRGPRX2 are E164 and D184 (pink color); amino acids involved in G protein coupling are V123, I225 and Y279 (green color); and amino acids involved in phosphorylation are S313, T321, S325, S327 and S328 (red color). (B) A predicted 3D homology model of MRGPRX2 showing N-term, C-term and seven transmembrane helix [77].
Recent research on naturally occurring MRGPRX2 missense variants and single-nucleotide polymorphisms (SNPs) revealed the possible mechanism of variation in the HSR in individuals [86][88]. The SNP may change the structure of MRGPRX2, which predisposes some individuals to MRGPRX2 hyperactivation. Thirty SNPs have been identified in the coding regions of human MRGPRX2, in which two of the most common SNPs are involved in phenotype changes. Amino acid substitution from asparagine 62 to tryptophan (N62T) and from asparagine 16 to histidine (N6H) may change the cytoplasmic domain 1 (CPD1) of MRGPRX2 and extracellular domain 1 (ECD1), respectively [88]. The four naturally occurring MRGPRX2 missense variants—glycine165 glutamic acid, aspartic acid184histidine, tryptophan243arginine and histidine259tyrosine (G165E, D184H, W243R, and H259Y)—failed to respond to MRGPRX2 agonists (SP, hemokinin-1, human β-defensin-3, and Icatibant) [88]. In another study, a similar group of potential missense variants was identified, with gain and loss of MRGPRX2-dependent SP-induced MC degranulation [86].
The missense variants valine123phenylalanine, arginine138cysteine arginine141cysteine and valine282methionine (V123F, R138C, R141C, and V282M) were identified as loss of function phenotypes for SP-induced mast cell activation and degranulation [86]. On the other hand, the missense variants serine325leucine and leucine329glutamine (S325L and L329Q) were identified as gain of function phenotypes for SP-induced mast cell activation and degranulation [86]. These findings have important clinical implications in identification of patients with listed SNPs. The individuals with loss of function MRGPRX2 variants can be resistant, while those with gain of function variants can be more susceptible to MRGPRX2 ligands. Prior identification of such variants may be helpful in categorizing susceptible individuals who can show drug-induced MC degranulation and HSRs. However, more studies are warranted to support these possibilities.
MRGPRX2 is a class A GPCR which activates the downstream signaling pathway via activation of trimeric G protein. Upon ligand binding to the N-terminal region of MRGPRX2, the Gαq protein is activated and leads to Ca2+ influx followed by MC degranulation [86][91]. Activation of the Gαq protein leads to activation of the phospholipase C-γ (PLCγ) pathway, which was inhibited by a PLCγ inhibitor U-73122 [48][92] Moreover, MRGPRX2 has also been reported to activate the PTx-sensitive Gαi pathway. PTX blocked the Ca2+ mobilization and MC degranulation activity of MRGPRX2 agonists such as compound 48/80 [93], SP [94], and Icatibant [95]. Interestingly, some MRGPRX2 agonists have shown a biased signaling mechanism via the beta-arrestin pathway [95]. It is of great interest to explore the beta-arrestin pathway of MRGPRX2, which causes biased signaling [87][95]. These findings suggested the involvement of Gαq, Gαi and beta-arrestin in MRGPRX2 signaling. MRGPRX2 activation can trigger several downstream signaling pathways, which contributes to ongoing allergic and inflammatory reactions [96]. MRGPRX2 agonists such as human beta defensins and LL-37 have been reported to activate the MAPK/ERK, p38, and JNK pathways and increased the release of IL-31, PGE2, and leukotriene C4 [96][97]. A recent study reported the involvement of the Nrf2 pathway in MRGPRX2-mediated MC degranulation and pseudo-allergic reactions [98]. Indeed, more studies are warranted to understand the downstream signaling pathways of MRGPRX2.
A plethora of MC-degranulating compounds such as peptide toxin mastoparan, neuropeptides and compound 48/80 have long been known to induce MC degranulation via an unknown GPCR. Another mechanism of receptor-independent or direct activation of G proteins (Gαi2 and Gαi3) to induce downstream signaling for degranulation has also been reported [43][44]. In recent years, MRGPRX2 has been identified as the unknown GPCR which binds with several compounds and induces MC degranulation [27][45]. Unlike other GPCRs that have selective and limited agonists, MRGPRX2 is known to have many agonists, including cationic amphiphilic drugs, insect venom chemical components, antimicrobial peptides, secreted eosinophil products, neuropeptides, small compounds, and natural compounds (Figure 3).
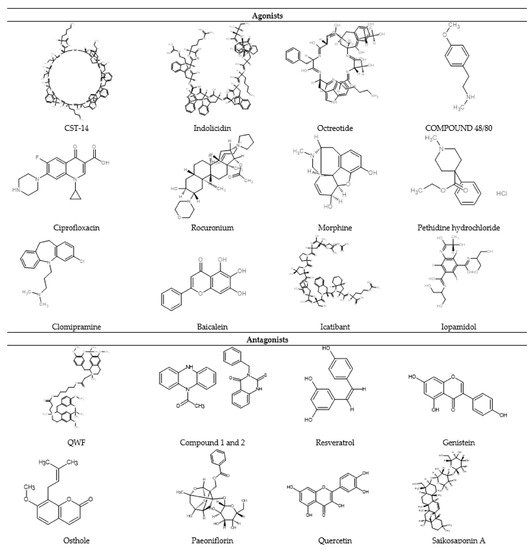
Figure 3. 2D chemical structure of some MRGPRX2 agonists and antagonists.
Moreover, MRGPRX2 has been reported as a low-affinity and low-selectivity receptor, which allows its interaction with diverse ligands (Figure 1). For ligand binding and G protein coupling, MRGPRX2 ligands use the conserved residues in their transmembrane (TM) domains and carboxyl-terminus Ser/Thr residues, respectively. In the first demonstration of non-IgE-mediated MC degranulation via MRGPRX2, the basic secretagogues such as peptides and compound 48/80 have shown a concentration -dependent increase in reporter gene expression in MRGPRX2-expressing PC12 cells and Ca2+ mobilization in MRGPRX2 expressing human embryonic kidney (HEK293) cells [45]. Host defense peptides (HDPs) such as LL-37 are potent MC chemoattractants and can induce degranulation [99]. HDPs modulate TLR4 on MCs and increase the expression of TLR4, which may enhance their ability to detect invading pathogens [100][101].
Epithelium and neutrophil-derived HDPs (hBDs and LL-37) have recently been reported to activate human MCs via MRGPRX2 [50][102][103]. This activation leads to MC degranulation but also the release of significantly less pro-inflammatory cytokines [56]. However, keratinocytes show a different response against LL-37 challenge, possibly via the activation of MRGPRX3/4. This activation leads to degranulation with a very high generation of pro-inflammatory cytokines and promotes wound healing [79][97]. Thus, the interaction between keratinocytes and mast cells via MRGPRX3/4 and MRGPRX2, respectively, has demonstrated a potential pathway involved in cutaneous host defense and wound healing. In addition, SP, a known endogenous ligand of MRGPRX2, has been shown to play a role in neurogenic inflammation and pain associated with wound healing by recruiting innate immune cells to the injury site [74]. In the following section, we have summarized the MRGPRX2 agonists into different groups according to their chemical nature such as peptides and non-peptides (drugs and natural compounds) (Table 1).
Table 1. Summary of the MRGPRX2/MRGPRB2 agonists (peptides, non-peptides and natural compounds).
|
SN |
Agonist |
Major Findings |
References |
|---|---|---|---|
|
Peptide Agonist |
|||
|
1 |
CST-14 |
|
|
|
2 |
Substance P |
|
|
|
3 |
Somatostatin |
|
|
|
4 |
PACAP |
|
[45] |
|
5 |
VIP |
|
[45] |
|
6 |
LL-37 |
|
|
|
7 |
β-defensins |
|
[103] |
|
8 |
Indolicidin |
|
[45] |
|
9 |
PAMP 20 |
|
|
|
10 |
Mastoparan |
|
|
|
11 |
Human eosinophil granules |
|
|
|
12 |
Proteases |
|
|
|
13 |
AG-30/5C |
|
[95] |
|
14 |
AMP-IBP5 |
|
[48] |
|
15 |
GnRHR agonist and antagonist |
|
[72] |
|
Non-peptide agonist |
|||
|
1 |
Compound 48/80 |
|
|
|
2 |
Fluoroquinolone antibiotics (ciprofloxacin) |
|
|
|
3 |
NMBAs (rocuronium, mivacurium, and atracurium) |
|
|
|
4 |
Opioids (morphine, pethidine chloride, dextrorphan, levorphanol) |
|
|
|
5 |
Antidepressant drugs (clomipramine paroxetine and desipramine |
|
[115] |
|
6 |
Others (Icatibant, iopamidol, vancomycin, baicalein, and sinomemnin) |
|
|