1000/1000
Hot
Most Recent

Carbon dioxide (CO2), a vital molecule of the carbon cycle, is a critical component in living organisms’ metabolism, performing functions that lead to the building of compounds fundamental for the life cycle. In all living organisms, the CO2/bicarbonate (HCO3−) balancing is governed by a superfamily of enzymes, known as carbonic anhydrases (CAs, EC 4.2.1.1). CAs catalyze the pivotal physiological reaction, consisting of the reversible hydration of the CO2 to HCO3− and protons.
The fossil fuel use, land-use changes as well as the natural carbon sources on land and in oceans has drastically influenced the growth rate of atmospheric CO2 [1]. In the last twenty years, human CO2 emissions have been enormously accelerated, considering the overall rise in energy consummation, the greater use of coal to produce energy, increased consumption per capita, and population development [1]. Changes in atmospheric CO2 mirrors the balance between carbon emissions due to human activity and the dynamics of many terrestrial and ocean processes that remove or emit CO2 [2]. The increased CO2 favors the photosynthetic activity of plants and increases carbon storage in the plants themselves and soil [2]. Cheng et al. believe that carbon storage seems to be mainly due to fungi (the so-called Arbuscular Mycorrhizal Fungi), which settle near the roots of about 80% of plant species, providing essential nutrients to the plants in exchange for carbohydrates [3]. CO2, a key molecule of the carbon cycle, is a critical component in the metabolism of living organisms, performing functions which lead to the building of compounds fundamental for the life cycle [4]. At the same time, CO2 is a waste product since it is the end-product of respiration, reaching a concentration of about 5% in the human bloodstream and tissues. This concentration is higher than the level of CO2 in the atmosphere (about 0.036%) [4]. Intriguingly, opportunistic and pathogenic fungi sense the CO2 difference, which influences fungal differentiation, determining the expression of those fungal features essential for virulent or non-virulent traits [5]. Pathogenic fungi are responsible for superficial diseases such as dermatophytes (infections of skin, hairs, and nails), or may lead to systemic illness (candidiasis, aspergillosis, cryptococcosis, mucormycosis, and others) [6][7][8][9]. Two molecules are crucial for the fungal CO2-sensing: (1) bicarbonate (HCO3−), which is a meiosis- and sporulation-promoting ion [10], and (2) adenylyl cyclase (AC) that is involved in the spore formation [11][12][13]. In Cryptococcus, bicarbonate directly activates a soluble form of AC necessary for the polysaccharide capsule formation [14][15][16][17]. AC catalyzes cyclic AMP (cAMP) synthesis, an essential intracellular regulatory molecule, which permits a link between CO2/HCO3−/pH chemosensing and signaling [18]. cAMP signaling is involved in many metabolic reactions as well as in fungal development and virulence [19]. The fungal virulence of Cryptococcus neoformans, the etiological agent responsible for cryptococcosis [9], is induced by high CO2 levels in mammalian hosts, causing the production of a massive polysaccharide capsule, which inhibits phagocytosis and impairs cell-mediated immune response [14][15][20]. However, it also true that other factors than high CO2 levels contribute to inhibit mating in the host as demonstrated by the use of a murine model of cryptococcosis. The carbonic anhydrase mutants for Can1 and Can2 (the two CAs encoded by the genome of C. neoformans) were both as virulent as wild type (wt), and quantitative measurements of fungal burden demonstrated that the Can2 mutant proliferates equivalently to the wt strain in the lungs and brain of infected animals [20]. In Candida albicans, the CO2 levels, through the relationship of bicarbonate, adenyl cyclase and cAMP, influence the growth of filamentous structures (hyphae), which are associated with the fungal virulence, adherence, secretion of hydrolases, and cell death in the hosts [19][21][22][23]. Twenty-six thiazolidines against several Candida spp. and Gram-positive and Gram-negative bacteria were tested. Although lacking significant antibacterial activity, the tested compounds exhibited selective antifungal activity with an equal potency to fluconazole and clotrimazole. Interestingly, CA was considered the putative target that could mediate the antifungal effects of these compounds [24].
Cloning of the genomes of several pathogenic and non-pathogenic fungi provided the opportunity to identify a superfamily of ubiquitous metalloenzymes, known as carbonic anhydrases (CAs, EC 4.2.1.1), which catalyze a pivotal physiological reaction, consisting of the reversible hydration of the carbon dioxide to bicarbonate and protons [25][26][27][28][29][30][31]. The spontaneous reversible CO2 hydration reaction in the absence of the catalyst occurs very slowly with a rate constant of 0.15 s−1, which arrives at 50 s−1 for the reverse reaction of bicarbonate dehydration at the physiological pH [31]. CA increases the velocity of the CO2 hydration reaction up to 104-106-fold [31].
The CA superfamily is ubiquitously distributed in all living organisms and classified into eight CA classes (α, β, γ, δ, ζ, η, θ, and ι). Their distribution is quite varied from plants, animals, bacteria, and archaea. [25][26][27][28][29]. The genome of mammals, for example, encodes only for the α-CA class, of which 15 isoforms have been identified, which accomplish specialized functions in various tissues and organs [32][33][34][35][36]. In plants, α and β-CAs actively participate in photosynthesis and biosynthetic reactions associated with it, as well as in some aforementioned processes [37]. In Bacteria, Archaea, and cyanobacteria, α, β, γ, and ι -CA classes are present. Their role is to balance the CO2/HCO3− concentration ratio and a role in the carbon dioxide fixation [29][30][31][37][38][39]. Marine diatoms encode for α- δ-,ζ-, θ- and ι-CAs, which are involved in carbon dioxide fixation and metabolism [40][41][42]. In protozoa have been detected α- and η-CAs. Probably, the η-CA-class, recently discovered, has a pivotal role in de novo purine/pyrimidine biosynthetic pathways [43].
The fungal CO2-sensing, related to the CO2/HCO3−/pH-sensing, is directly stimulated by HCO3− produced in a CA-dependent manner. In the fungal kingdom, the typical CA class identified is represented by β-class, and the majority of fungi encode at least one β-CA [13][44][45]. The genomes of basidiomycetous and hemiascomycetous yeasts encode only for β-CAs. In contrast, most filamentous ascomycetes contain multiple β-CA genes and, in some of them, it is possible to find genes encoding for α-CAs [13][44][45]. Here, some examples demonstrating that CAs are abundant in fungi and yeasts (the last are microscopic fungi consisting of solitary cells that reproduce by budding) as reported in the following examples. Saccharomyces cerevisiae, Candida albicans, and Candida glabrata have only one β-CA, whereas multiple copies of β-CA and α-CA-encoding genes were reported in other fungi [44][45]. Recently, it has been evidenced that CAs play an important role in fungal pathogen sensing and the control of sexual growth [44][45]. The β-CAs identified in Candida albicans and Candida glabrata indicated with the acronyms CaNce103 and CgNce103, respectively, are necessary for the development of these fungi in environments characterized by low-oxygen conditions, such as the skin [44][45]. The CA (Can2) encoded by the genome of Cryptococcus neoformans allows the growth of the yeast in its natural habitat. It is relevant to note how the link between AC, cAMP signaling, and CO2/HCO3− sensing is conserved in most fungi since it is an essential mediator of fungal metabolism and pathogenesis [13][44][45]. Again, the gene Nce103 identified in the genome of Saccharomyces cerevisiae encodes for a β-CA (ScCA), which is involved in the production of the bicarbonate essential for the enzyme catalyzing carboxylation reactions, such as the pyruvate carboxylase (PC), acetyl-CoA carboxylase (ACC), carbamoyl phosphate synthase (CPSase), and phosphoribosylaminoimidazole (AIR) carboxylase [46][47].
In 2009, the first crystal structure of the β-CA encoded in the genome of a fungus, i.e., Cryptococcus neoformans, was reported by Schlicker and coworkers [45]. It showed a dimeric organization similar to that found in the CA belonging to the plant-type β-class (the two cysteines and a histidine responsible for zinc coordination are conserved in the active site of such enzymes). Intriguingly, a Can2 (acronym used for the CA from C. neoformans) three-dimensional structure showed a peculiar N-terminal extension, which interacts with the entrance of the catalytic pocket of the dimer. The N-terminus is an internal regulator or an interaction site for a regulatory protein, affecting the Can2 activity [45]. It can be considered a switch for the activation/inactivation of the protein, which is regulated by physiological factors, like pH, small molecule, or proteins. [45].
In 2011, the structure of the first fungal α-CA was obtained, which was identified in the fungus Aspergillus oryzae [48]. Like for other α-CAs, the enzyme showed a central core formed by a twisted β sheet consisting of eight mostly anti-parallel strands. The ion cofactor resulted in an atom of Zn(II) coordinated to the three histidines of the catalytic pocket, which is at the bottom of a deep cavity in the protein center [48].
In 2014, the structures of two β-CAs belonging to the fungus Sordaria macrospora were resolved by X-ray crystallography [49]. Like Can2, the two β-CAs from S. macrospora showed a high structural similarity with plant-like β-CAs, but it was assembled in a tetrameric and not a dimeric form. The two CAs (CAS1 and CAS2) were distinguished for the type of conformations they assumed: CAS1 resulted in the open “type- I” conformation, while the CAS2 adopted a close “type-II” conformation [49]. Finally, between 2020 and 2021, CafA and CafB, two of the four β-CAs encoded by the genome of the fungus Aspergillus fumigatus, were crystallized and the structure resolved at 1.8 and 2.0 Å, respectively [50][51]. The catalytic sites of CafA and CafB look similar to those of other β-CAs. CafA showed the typical open conformation. Surprisingly, CafB revealed a unique active site at a low pH or in an oxidative environment, resulting in an inactive enzyme, with a disulfide bond formed by the two zinc-ligating cysteines [50]. Of course, CafB also adopts the typical active/inactive configurations in which a conserved aspartic acid is implicated in switching the enzyme in its open/closed state [52].
The first antimicrobial drug widely used in clinical settings was Prontosil [53], a sulfanilamide prodrug, which is isosteric/isostructural with p-aminobenzoic acid (PABA), the substrate of dihydropteroate synthase (DHPS) [54][55]. After sulfanilamide, a range of analogs, the sulfa drug class, are still used as antibacterials, even if many of them show substantial drug resistance issues. Sulfa drugs are derived from sulfonamides, and the presence of primary sulfonamide moieties in sulfanilamide characterized most of the investigated CAIs until recently [32][56][57][58]. Primary sulfonamides/sulfamates/sulfamides possess the general formula R-X-SO2NH2, where R can be an aromatic, heterocyclic, aliphatic, or sugar scaffold, X = nothing, O or NH (Figure 1).
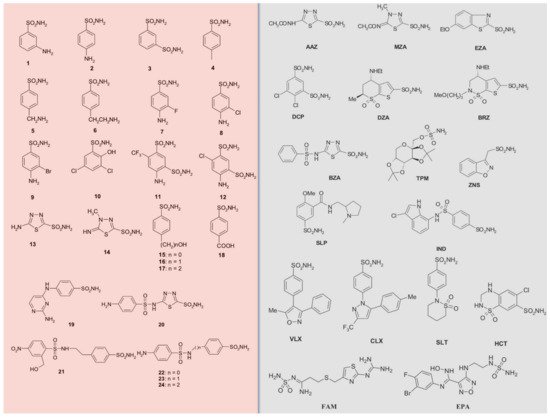
Figure 1. Sulfonamide/sulfamate/sulfamide of types 1–24 (pink background) and AAZ-EPA (gray background) investigated as fungal CA inhibitors. Legend: AAZ, acetazolamide; MZA, methazolamide; EZA, ethoxzolamide; DCP, dichlorophenamide; DZA, dorzolamide; BRZ, brinznolamide; BZA, benzolamide; TPM, topiramate; ZNS, zonisamide; SLP, sulpiride; IND, indisulam; VLX, valdecoxib; CLX, celecoxib; SLT, sulthiame; HCT, hydrochlorothiazide; FAM, famotidine; EPA, epacadostat.
Most of the sulfonamides acting as CAIs bind Zn (II) in a tetrahedral geometry, showing an extended network of hydrogen bonds with the enzyme amino acid residues, as seen by the enzyme-inhibitor X-ray crystallographic data [32][58][59]. The aromatic/heterocyclic part of the inhibitor interacts with the hydrophilic and hydrophobic residues of the catalytic cavity [32][58]. Compounds containing -SO2NH2 group, including clinically licensed drugs, are generally considered CAIs [27][60][61][62][63][64][65][66][67][68][69][70][71][72][73][74][75]. Some examples include: AAZ, MZA, EZA, and DCP, which are systemically acting antiglaucoma CAIs; DZA and BRZ are antiglaucoma agents; BZA belongs to the same pharmacological class; ZNS, SLT, and TPM are antiepileptic drugs; and SLP and IND, with COX2 selective inhibitors CLX and VLX. The diuretic hydrochlorothiazide (HCT) is also known to act as a CAI [35][76][77]. FAM is a competitive histamine H2-receptor antagonist [76], and EPA is an inhibitor of the heme-containing enzyme, indoleamine 2,3-dioxygenase-1 (IDO1), but they also act as CAIs [77] (see Figure 1). Table 1 shows selected inhibition data with some of these compounds against selected fungal CAs.
Table 1. Inhibition data of human isoenzymes (CA I and CA II) and fungal CAs (MreCA, MgCA, CAS1, CAS2, CAS3; Figure 1) by a stopped-flow CO2 hydrase assay. The other fungal CAs have been previously reviewed by Elleuche and Poggeler (see references [78][79]).
| Inhibitor | KI (nM) * | ||||||
|---|---|---|---|---|---|---|---|
| hCA I 1 (α-CA) |
hCA II 1 (α-CA) |
MgCA 2 (β-CA) |
MreCA 2 (β-CA) |
CAS1 1 (β-CA) |
CAS2 1 (β-CA) |
CAS3 1 (β-CA) |
|
| 1 | 28,000 | 300 | 980 | 412 | 361 | 386 | 90 |
| 2 | 25,000 | 240 | 24.5 | 462 | 144 | 3480 | 84 |
| 3 | 79 | 8 | 15.2 | >10,000 | 225 | 3630 | 83 |
| 4 | 78,500 | 320 | 674 | 404 | 47.1 | 6900 | 560 |
| 5 | 25,000 | 170 | 17.4 | >10,000 | 323 | 8720 | 726 |
| 6 | 21,000 | 160 | 7.9 | >10,000 | 241 | 7650 | 441 |
| 7 | 8300 | 60 | 11.6 | 459 | 43.2 | 7360 | 585 |
| 8 | 9800 | 110 | 12.1 | >10,000 | 79.6 | 9120 | 2078 |
| 9 | 6500 | 40 | 34.9 | >10,000 | 580 | 12,000 | 712 |
| 10 | 7300 | 54 | 54.3 | >10,000 | >50,000 | 23,500 | 350 |
| 11 | 5800 | 63 | 9 | 676 | 890 | 18,700 | 235 |
| 12 | 8400 | 75 | 9.2 | >10,000 | 3350 | >50,000 | 90 |
| 13 | 8600 | 60 | 7900 | >10,000 | 8650 | 48.1 | 88 |
| 14 | 9300 | 19 | 8500 | >10,000 | 7215 | 280 | 94 |
| 15 | 5500 | 80 | 23.6 | >10,000 | 3160 | 143 | 605 |
| 16 | 9500 | 94 | 10.4 | 651 | 4520 | 92.5 | 82 |
| 17 | 21,000 | 125 | 6.3 | >10,000 | >50,000 | 390 | 507 |
| 18 | 164 | 46 | 6.8 | >10,000 | 4443 | 3250 | 226 |
| 19 | 109 | 33 | 3500 | 779 | 475 | 6760 | 91 |
| 20 | 6 | 2 | 23.4 | 91 | 363 | 9880 | 85 |
| 21 | 69 | 11 | 11.8 | 740 | 4550 | 4060 | 95 |
| 22 | 164 | 46 | 9.4 | 374 | 1985 | 25,200 | 85 |
| 23 | 109 | 33 | 4530 | >10,000 | 282 | >50,000 | 89 |
| 24 | 95 | 30 | 256 | >10,000 | 294 | >50,000 | 84 |
| AAZ | 250 | 12 | 7600 | 10 | 445 | 816 | 94 |
| MZA | 50 | 14 | 7455 | 390 | 421 | 8140 | 91 |
| EZA | 25 | 8 | 3800 | 379 | 440 | 3170 | 95 |
| DCP | 1200 | 38 | 34.6 | 306 | 1220 | 5790 | 73 |
| DZA | 50,000 | 9 | 7900 | 81 | 360 | 742 | 274 |
| BRZ | 45,000 | 3 | 8400 | 70 | 451 | 739 | 61 |
| BZA | 15 | 9 | 48.2 | 715 | 2115 | 410 | 54 |
| TPM | 250 | 10 | 146 | 383 | 414 | 673 | 363 |
| ZNS | 56 | 35 | 765 | >10,000 | 1820 | 1885 | 710 |
| SLP | 1200 | 40 | 32 | 485 | 1715 | 670 | 493 |
| IND | 31 | 15 | n.d. | 87 | 4240 | 216 | 94 |
| VLX | 54,000 | 43 | 3150 | 77 | 4425 | 3730 | 831 |
| CLX | 50,000 | 21 | 3480 | 140 | 2513 | 857 | 669 |
| SLT | 374 | 9 | n.d. | 67 | 3210 | 496 | 4838 |
| SAC | 18,540 | 5959 | n.d. | 620 | 5280 | 7075 | 191 |
| HCT | 328 | 290 | n.d. | 850 | 3350 | 6680 | 545 |
| FAM | n.d. | n.d. | n.d. | >10,000 | n.d. | n.d. | n.d. |
| EPA | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
* Errors were in the range of ±5–10% on three different assays. 1 From reference [80] and [81]; 2 From reference [82]; n.d.: not detected.
These CA inhibitors include inorganic anions as well as several more complex species such as carboxylates, which are in fact organic anions [58][59]. Anions may bind either in the tetrahedral geometry of the metal ion or as trigonal–bipyramidal adducts [83]. Anion inhibitors show KIs in a millimolar range, diversely from the sulfonamides mentioned above, which is generally showed as KIs in the micro to nanomolar range. But their investigation as CA inhibitors offers the possibility to better understand the inhibition/catalytic mechanisms of the CAs, for improving the design of novel types of inhibitors that may have clinical applications [58][59]. A list of anions and their CA inhibitory action against selected fungal CAs is shown in Table 2.
Table 2. Inhibition constants obtained using anionic inhibitors versus the α-CA isozymes of human origin (hCA I and hCA II), and Table 1. CAS2, CAS3; for the acronyms, see the text) by a stopped flow CO2 hydrase assay.
| Anion | KI (mM) * | ||||||
|---|---|---|---|---|---|---|---|
| hCA I 1 (α-CA) |
hCA II 1 (α-CA) |
MgCA 2 (β-CA) |
MreCA 2 (β-CA) |
CAS1 1 (β-CA) |
CAS2 1 (β-CA) |
CAS3 1 (β-CA) |
|
| F− | >300 | >300 | 7.13 | >50 | >100 | >100 | >100 |
| Cl− | 6 | 200 | 7.98 | >50 | 9.2 | >100 | >100 |
| Br− | 4 | 63 | 18.6 | >50 | 9.3 | >100 | >100 |
| I− | 0.3 | 26 | 8.73 | 8.6 | 8.6 | 7.7 | 9.9 |
| CNO− | 0.0007 | 0.03 | 6.81 | >50 | 0.9 | 0.82 | 3.2 |
| SCN− | 0.2 | 1.60 | 8.39 | >50 | 5.4 | 5.6 | 7.3 |
| CN− | 0.0005 | 0.02 | 7.19 | >50 | 0.94 | 0.75 | 8.7 |
| N3− | 0.0012 | 1.51 | 45.2 | >50 | >100 | 6.1 | 7.2 |
| NO2− | 8.4 | 63 | 7.56 | >50 | >100 | >100 | 8.3 |
| NO3− | 7 | 35 | 8.13 | 9 | >100 | >100 | 8.5 |
| HCO3− | 12 | 85 | 0.59 | 0.86 | 3.3 | 7.3 | >100 |
| CO32− | 15 | 73 | >100 | >50 | >100 | 8.8 | 8 |
| HSO3− | 18 | 89 | >100 | >50 | 3.3 | 7.3 | >100 |
| SO42− | 63 | >200 | 19.5 | >50 | >100 | 4.8 | >100 |
| HS− | 0.0006 | 0.04 | 11.9 | >50 | 0.89 | 8.5 | 8.3 |
| SnO32− | 0.57 | 0.83 | 5.07 | 0.56 | 4.3 | 0.92 | 7.9 |
| SeO42− | 118 | 112 | 7.41 | 1.7 | 2.4 | 9.2 | 3.4 |
| TeO42− | 0.66 | 0.92 | 5.75 | 0.56 | 2.5 | 6.3 | 8.1 |
| OsO52− | 0.92 | 0.95 | 6.16 | 8.5 | n.d. | n.d. | n.d. |
| P2O74− | 25.77 | 48.50 | 6.03 | >50 | 3.1 | 0.96 | >100 |
| V2O74− | 0.54 | 0.57 | 6.89 | >50 | >100 | 1.4 | >100 |
| B4O72− | 0.64 | 0.95 | 8.45 | 0.4 | 6.7 | 6.9 | 5.9 |
| ReO4− | 0.11 | 0.75 | 16.7 | >50 | 8.2 | >100 | 8.8 |
| RuO4− | 0.101 | 0.69 | 8.82 | 7.4 | 3.9 | >100 | 9.2 |
| S2O82− | 0.107 | 0.084 | >100 | >50 | 5 | >100 | >100 |
| SeCN− | 0.085 | 0.086 | 1.73 | 0.65 | 2.9 | 9.3 | 7.1 |
| CS32− | 0.0087 | 0.0088 | 1.77 | 0.92 | 0.79 | >100 | 8.6 |
| Et2NCS2− | 0.00079 | 0.0031 | 0.30 | 0.075 | 0.38 | 0.93 | 0.89 |
| CF3SO3− | n.d. | n.d. | 2.28 | 4.5 | n.d. | n.d. | n.d. |
| PF6− | n.d. | n.d. | 6.47 | 3.9 | n.d. | n.d. | n.d. |
| ClO4− | >200 | >200 | >100 | 9.2 | >100 | >100 | >100 |
| BF4− | >200 | >200 | >100 | 383 | >100 | >100 | >100 |
| FSO3− | 0.79 | 0.46 | 4.06 | >50 | 0.93 | 8.4 | >100 |
| NH(SO3)22− | 0.31 | 0.76 | 21.4 | >50 | 0.88 | 9.2 | >100 |
| H2NSO2NH | 0.31 | 1.13 | 0.094 | 0.72 | 0.084 | 0.048 | 0.094 |
| H2NSO3H | 0.021 | 0.39 | 0.083 | 7.7 | 0.069 | 0.072 | 0.095 |
| Ph-B(OH)2 | 58.6 | 23.1 | 0.089 | 8.7 | 0.009 | 0.056 | 0.097 |
| Ph-AsO3H2 | 31.7 | 49.2 | 0.090 | 0.83 | 0.035 | 0.054 | 0.091 |
* Errors were in the range of ±5–10% on three different assays. 1 From reference [84]; 2 From reference [85]; n.d.: not detected.
Anions inhibit fungal CAs by coordinating to the metal ion within the enzyme active site, as exemplified in Figure 2 for Can2 complexed with acetate. As for all β-CAs, Can2 is a dimer and the active site contains amino acid residues from both monomers. The zinc ion is coordinated as shown in Figure 2, by Cys68, His124, and Cys127, whereas acetate is the fourth zin ligand, being coordinated monodentately by one of the oxygen atoms. The same type of inhibition mechanism is valid for all anions shown in Table 2, although few X-ray crystal structures of such adducts are available to date [45].
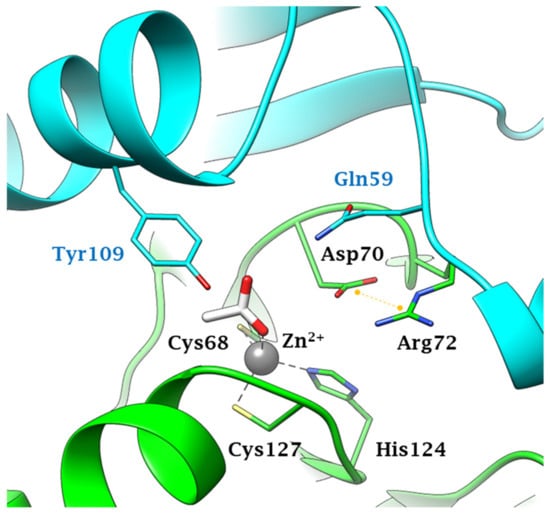
Figure 2. Active site view of Can2 (pdb 2W3N) complexed to the anion inhibitor acetate [45]. Protomers A and B are colored green and cyan respectively. Residues from protomers A and B are labeled black and light blu, respectively. The Zn2+ ion, represented as a grey sphere, is coordinated by two cysteines and one histidine residue from monomer A and by one acetate ion as a ligand. The salt bridge in the Asp-Arg dyad is represented as a yellow dashed line.