1000/1000
Hot
Most Recent

Multi-strain probiotics are composed of more than one species or strains of bacteria and sometimes, including some fungal species with benefits to human and animals’ health. The mechanisms by which multi-strain probiotics exert their effects include cell-to-cell communications, interactions with the host tissues, and modulation of the immune systems.
The Food and Agriculture Organization/World Health Organization (FAO/WHO) working committee on probiotics defined probiotics as “live microorganisms which when administered in adequate amounts confer health benefits on the host” [1]. The use of probiotics is increasing due to consideration as a suitable option following restrictions on antibiotics as growth promoters in the livestock industries by many countries [2]. There are different forms of probiotics preparations, and sometimes, their efficacy depends on whether they are single- or multi-strain preparations [3]. Compared to single-strain preparations, multi-strain probiotics contain more than one strain of the same species, genera, or multiple genera and sometimes including both bacteria and fungi (Saccharomyces species) [4]. Some single-strain probiotics are beneficial in alleviating gastrointestinal-tracts-associated diseases [5]. However, previous in-vitro studies showed that some multi-strain probiotics could exhibit better inhibitory effects on entero-pathogens [6] and enhanced benefits by combining effects of different strains compared to their single-strain preparations [7].
The mechanism of probiotics' actions is the various means by which they exert their beneficial effects on the host, including immune modulation, stimulation/modulation of gut microbiota, stimulation of digestive enzymes, displacement of pathogens, and production of bioactive compounds [8][9][10]. The gut-associated actions are the principal effects of probiotics, also regarded as the basis of other health benefits [11] as summarized in Figure 1.
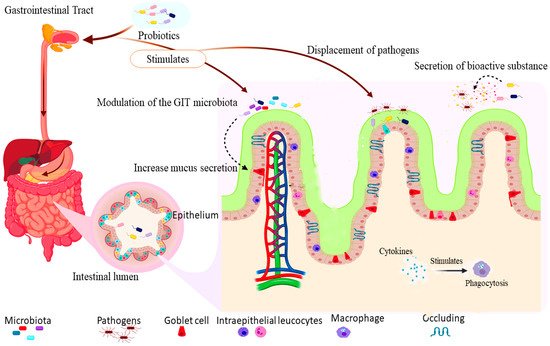
Figure 1. shows the mechanism of actions of probiotics: the intake of probiotics stimulates an increase in the secretion of mucus by goblet cells, mobilization of intraepithelial leucocytes, and tightening of the tight junctions to protect against the invasion of pathogens. The increase in mucus secretion and improvement of gut microbiota enhances competitive displacement and inhibition of pathogens adhesion to the gut epithelial surface. Furthermore, the action of bioactive substances such as lysozyme and cytokines stimulate phagocytosis by macrophages.
Different randomized control clinical trials revealed that some specific probiotics are useful in the therapeutic management of gastrointestinal (GI) illnesses like inflammatory bowel disease (IBD) [12], irritable bowel syndrome (IBS), and pouchitis [13][14]. The administration of multi-strain probiotics containing different Lactobacilli species, Streptococcus and Bifidobacterium, to patients who have systemic sclerosis alleviates the symptoms of gastrointestinal reflux and increased microbial alpha diversity group [14] (Table 1).
| Probiotics Mixture | Conditions | Mechanism of Actions | References |
|---|---|---|---|
| B. bifidum W23, B. lactis W52, L. acidophilus W37, L. brevis W63, L. casei W56, L. salivarius W24, Lactococcus lactis W19 and L. lactis W58 | Endotoxins | Improvement of endothelial barrier, inhibition of mast cell, activation of proinflammatory cytokines, and decrease endotoxin | [15] |
| L. acidophilus, L. casei, B. bifidum, and L. fermentum | Cognitive function in Alzheimer’s disease | [16] | |
| L. paracasei DSM 24,733, L. plantarum DSM 24,730, L. acidophilus DSM 24,735, and L. delbrueckii subspecies bulgaricus DSM 24,734), Bifidobacteria (B. longum DSM 24,736, B. breve DSM 24,732, and B. infantis DSM 24,737), and Streptococcus (S. thermophilus DSM 24,731) | Systemic sclerosis-associated gastrointestinal disease | Improvement of GI reflux and intestinal microbiota alpha diversity | [17] |
| L. acidophilus LaVK2 and B. bifidum Bbvk3 | Dextran sodium- sulphate salt-induced ulcerative colitis in mice | Reduction in myeloperoxidase activity, levels of TNF-α, IL-6, and IFN-γ | [18] |
| L. bulgaricus 151 and S. thermophilus MK-10 | Dextran sodium- sulphate salt-induced colitis | Modulation of intestinal microbiota, decrease the content of putrefactive short-chain fatty acid, enhanced production of cytokines. | [19] |
| B. bifidum (KCTC 12199BP), B. lactis (KCTC 11904BP), B. longum (KCTC 12200BP), L. acidophilus (KCTC 11906BP), L. rhamnosus (KCTC 12202BP) and S. thermophilus (KCTC 11870BP) | Irritable Bowel Syndrome (IBS) | Alleviation of IBS symptoms and improvement of intestinal microbiota | [20] |
| B. longum and L. casei strain Shirota | Treatment of obesity | Decreased weight and triglyceride in rats fed with the high-fat diet. | [21] |
| S. boulardii, L. acidophilus, L. plantarum, B. lactis | IBS associated with bacterial overgrowth and constipation | Improvement in bloating, and pain associated with constipation | [22] |
| L. plantarum, B. breve, and L. fermentum | high-dietary fat-induced obesity and E. coli challenged | Causes reduced Lipopolysaccharide and IL-1β, improved the structure of intestinal flora and increased the fecal short-chain fatty acid (SCFA) content | [23] |
A multi-strain probiotic containing different Lactobacillus strains hinders the adhesion of E. coli and E. faecalis to the bladder cell lines, unlike the single-probiotics preparations [24] (Table 2).
| Multi-Strain Probiotics Isolates | Pathogenic Bacteria | Host | References |
|---|---|---|---|
| B. subtilis and L. mesentroides | Vibrio cholereae | In-vitro agar diffusion test | [25] |
| L. plantarum F44, L. paracasei F8, B. breve 46 and B. lactis | Clostridium difficile | Mice | [26] |
| S. oralis and S. salivarius | Biofilm (S. aureus, S. epidermidis, S. pneumoniae, S. pyogenes, Propionibacterium acnes and Moraxella catarrhalis | Dogs | [27] |
| L. acidophilus LAP5, L. fermentum P2, P. acidophilus LS, and L. casei L21 | S. enterica subspecies Enterica | Chickens | [28] |
| L. acidophilus LA-5 and B. bifidum BB-12 | P. stomatis, P. multocida, P. canis, N. animaloris, and N. zoodegmatis | [29] | |
| P. acidilactici and S. cerevisiae boulardii | Enterotoxigenic E. coli (ETEC) F4 | Pigs | [30] |
| L. acidophilus NCIMB 30184, L. fermentum NCIMB 30226, L. plantarum NCIMB 30187, and L. rhamnosus NCIMB 30188 | Pathogenic E. coli and E. faecalis | [24] | |
| S. cerevisiae, E. faecium, L. acidophilus and Bacillus subtilis | E. coli | Chickens (broilers) | [31] |
| L. acidophilus NCIMB 30184, L. rhamnosus NCIMB 30188, L. plantarum NCIMB 30187, L. delbrueckii ssp. bulgaricus NCIMB 30186, L. casei NCIMB 30185, L. lactis NCIMB 30222, L. salivarius NCIMB 30225, L. fermentum NCIMB 30226, L. helveticus NCIMB 30224, B. bifidum NCIMB 30179, B. breve NCIMB 30180, B. infantis NCIMB 30181, B. longum NCIMB 30182, S. thermophilus NCIMB 30189 B. subtilis NCIMB 30223 | S. typhimurium, C. difficile | In-vitro distal colon model | [32] |
| L. acidophilus NCIMB 30184, L. fermentum NCIMB 30188, L. plantarum NCIMB 30187 and L. rhamnosus NCIMB 30226 | E. faecalis NCTC 0075 and E. coli NCTC 9001 | In-vitro agar diffusion test | [6] |
| L. rhamnosus and L. reuteri | Vaginal coliforms and yeast | Human (female) | [33] |
| L. crispatus, L. salivarius, L. gallinarum, L. johnsonii, E. faecalis and B. amyloliquefaciens | Salmonella Enteritidis A9 | Chickens (broiler) | [34] |
| L. acidophilus, L. fermentum, L. plantarum and E. faecium | Salmonella enterica | Chickens (broiler) | [35] |
| B. amyloliquefaciens B-1895 and B. subtilis KATMIRA1933 | Inhibits Proteus mirabilis biofilm formation | Invitro | [36] |
| E. faecalis (strains NM815, and NM915) and E. faecium NM1015 | C. difficile infection | Mice | [37] |
| L. acidophilus (LA-5), and B. animalis subspecies Lactis (Bb12) | E. coli induced pyelonephritis | Sprague-Dawley rat | [38] |
| L. casei and E. faecium | Entamoeba invadens | Invitro | [39] |
| B. subtilis, L. acidophilus, P. acidilactici, P. pentosus, Saccharomyces pastorianus | Avian pathogenic E. coli and Salmonella Kentucky | White leg-horn chicks | [40] |
| L. gasseri and L. rhamnosus | Non-Candida albicans biofilm formation | In-vitro | [41] |
Multi-strain probiotics had proved beneficial for the treatment of dysentery in addition to the standard regimen with a marked reduction in the extent of bloody stooling and a decreased average length of hospital stay [42]. These effects were a result of the alteration of the microbial and metabolic activities within the gut, and which are enough to modify the disease process and pathological conditions [43].
Probiotics may be a potential alternative for improving gastrointestinal health and growth promotion in different animal species [44]. Based on these, the roles of probiotics in the various livestock sub-sectors, including poultry, aquaculture, piggery, and ruminant nutrition, were discussed as follows.
In poultry, the addition of probiotics derived from Lactobacillus, Bacillus [45], and Clostridium species to feed has a positive impact on the growth yield, feed digestion [46], immunity [47], meat quality [48], and coliforms bacterial count [44][49]. The administration of multi-strain probiotics (comprising of L. acidophilus LAP5, L. fermentum P2, P. acidophilus LS, and L. casei L21) to specific-pathogen-free (SPF) chicks infected with Salmonella enterica subspecies enterica decreases the abundance of proteobacteria of which Salmonella is a member [28].
The use of probiotics for health improvement has also found application in aquaculture. The addition of multi-strain probiotics in the feed of rohu (Labeo rohita) was revealed to stimulate cellulolytic and amylolytic enzymes secretions with improved growth output [50]. The multi-strain culture of B. subtilis, B. licheniformis, and lactobacilli probiotics significantly improves pacific white shrimps’ growth (Litopenaeus vannamei) and enhances non-specific immunity and the abundance of Bacillus to influence the intestinal microbiota [51].
The weaning period in piggery coupled with diets changes from simply digestible (milk) to solid feeds may result in intestinal perturbation, thereby causing diarrhea and a slow growth rate [52][53][54]. The ingestion of probiotic bacteria (like P. acidilactici) and yeast (S. cerevisiae boulardii) protect from microbial infection by enhancing intestinal defences and performance in different monogastric animals [55].
Some probiotics are suitable supplements in livestock feeds and may improve the rumen’s microbial ecosystem, enhance feed digestion, and restores gut microflora in diarrhea in ruminants [56]. The administration of lactobacilli probiotics enhances calves’ overall health status [57].
Some probiotics are prepared as synbiotics (prebiotics) along with other active substances for maximum physiological effects. Ingestion of synbiotics made of multi-strain probiotics (containing L. acidophilus strain T16, L. casei strain T2 and B. bifidum strain T1) and 800 mg inulin (HPX) by gravid women with gestational diabetes mellitus decrease the rate of caesarean section and hyperbilirubinemia and hospitalization of newborns [58]. Administration of synbiotics (containing multi-strain probiotics and prebiotics) may alleviate some digestive system conditions, sepsis, and death in preterm babies [59] (Table 3).
| Synbiotics | Actions | Host | References |
|---|---|---|---|
| L. acidophilus strain T16, L. casei strain T2) and B. bifidum strain T1 plus 800mg inulin (HPX) | decreased the incidence of cesarean section rate and newborn’s hyperbilirubinemia and hospitalization | Human (pregnant women) | [58] |
| L. acidophilus, L. rhamnosus, S. thermophilus, and L. delbrueckii subspecies Bulgaricus plus fluconazole | Enhance the treatment of Vaginal candidiasis caused Candida albicans | humans | [60] |
| L. plantarum, L. acidophilus, L. delbrueckii subspecies bulgaricus, B. bifidum, L. rhamnosus, E. faecium, S. salivarius subspecies thermophilus, Aspergillus oryza, and Candida pintolopesii plus Zinc | Enhances growth performance, better feed utilization, increase in villus height in the duodenum and ileum | Chicken (broiler) | [61] |
| Synbiotics A: Enterococcus sp., Pediococcus sp., Bifidobacterium sp., Lactobacillus sp. plus fructooligosaccharides Synbiotic B: L. acidophilus, L. casei, L. salivarius, L. plantarum, L. rhamnosus, L. brevis, B. bifidum, B. lactis, S. thermophilus, prebiotic inulin (chicory root extract), protease, amylase, cellulase, hemicellulase, lipase, papain and bromelain | Modulate the caecal microbiota without any effects on Salmonella Typhimurium shedding | Chickens (layers) | [62] |
| Probiotics; (L. rhamnosus, L. casei L. plantarum B. animalis) prebiotics (383 mg of fructooligosaccharides and 100 mg of galactooligosaccharides) | Improved gastrointestinal complications, sepsis, and mortality in premature infants | Preterm infants | [59] |
To maximize all the benefits of probiotics consumption, research should determine the specific mechanisms of actions of probiotics microbes for more specific applications in respective disease conditions. There is also the need to study and understand each probiotics strain’s best combination because some bacteria act synergistically, some additively, and some antagonistically. Additionally, the bioactive substances produced by some probiotics could be extracted to formulate supplements for use in specific conditions where individuals showed some reactions to the consumption of the whole-cells preparations. The harvesting and harnessing of the bioactive substances produced by individual constituents of mixed probiotics could also solve the challenges associated with the inconsistency of viable cells when live microbes are used. That will also enable large-scale production for commercialization. Finally, further studies in this direction could be an essential factor in the future research and development of multi-strain probiotics.