1000/1000
Hot
Most Recent

Ischemia reperfusion injury (IRI) is inevitable in kidney transplantation and negatively impacts graft and patient outcome. Reperfusion takes place in the recipient and most of the injury following ischemia and reperfusion occurs during this reperfusion phase; therefore, the intra-operative period seems an attractive window of opportunity to modulate IRI and improve short- and potentially long-term graft outcome. Commonly used volatile anesthetics such as sevoflurane and isoflurane have been shown to interfere with many of the pathophysiological processes involved in the injurious cascade of IRI.
During the process of kidney donation and transplantation a number of potentially harmful processes will inevitably occur, which affect the viability of the graft. The donor organ is, by definition, exposed to a period of ischemia which lasts until the kidney is re-connected to the circulation of the recipient. In the case of deceased kidney donation, additional injury occurs even before graft removal. The pro-inflammatory and pro-coagulatory systemic and local renal response to brain death in the case of deceased brain dead (DBD) donors and the variable and often extended period of warm ischemia and hypoxia in the case of deceased circulatory death (DCD) donors, result in further impairments, reducing post-transplant graft function and survival. These combined effects on the graft-to-be result in a cascade of renal damage that will reveal itself at the time of transplantation when the donor kidney is reperfused in the recipient and is called ischemia reperfusion injury (IRI). Typically, IRI manifests as immediate non-function of the graft with the need for dialysis treatment until the graft recovers from the insult and starts to function. This “secondary” recovery is called delayed graft function (DGF). In cases where the injury has been too extensive to repair the transplanted kidney and it will never recover nor regain function, this is called primary non function (PNF). DGF and PNF are clinically relevant problems. Both are associated with increased morbidity, patient anxiety, prolonged hospitalization, and additional diagnostic procedures and costs. Furthermore, DGF is associated with acute rejection (AR), and the combination of DGF and AR reduces graft and patient survival [1][2].
IRI consists of a complex pathophysiology in which several molecular pathways and signaling cascades are involved [3]. It is amongst others associated with dysfunctioning of the mitochondrial respiratory chain and uncontrolled formation of reactive oxygen species (ROS) during reperfusion, leading to opening of the mitochondrial permeability transition pores (mPTP) and release of danger-associated molecular patterns (DAMPs) into the intra- and extracellular space [4][5]. From here, several injury cascades are implicated, including the activation of cell death programs such as apoptosis and (regulated) necrosis and endothelial dysfunction with loss of the glycocalyx and transmigration of leucocytes into the interstitial space. The DAMPs released upon reperfusion will further harm the graft by binding to pattern recognition receptors (PRR) such as toll-like receptors (TLR) and receptors of the complement system, leading to activation of the innate and subsequently the adaptive immune system [3]. This will increase the immunogenicity of the graft, favoring T cell and antibody mediated rejection (ABMR) and the initiation or progression of interstitial fibrosis associated with chronic graft dysfunction. In addition, protective pathways in cells are activated upon IRI by means of activation of transcription factors such as hypoxia inducible factors (HIFs) involved in the regulation of genes associated with the metabolic cell cycle, angiogenesis, and cell survival [6].
Conditioning is a broad term generally used to describe strategies inducing biochemical changes that attenuate IRI. Dependent on timing of application of the strategy, it is referred to as pre- (before ischemia), per- (during ischemia), or post- (directly upon reperfusion) conditioning. It was first described in 1986 by Murry and colleagues [7]. They reported that subjecting the heart to four brief ischemic episodes, followed by reperfusion, preceding a prolonged ischemic insult, reduced myocardial infarct size by 75% in a canine model [7]. This phenomenon is called ischemic preconditioning (IPC). Subsequently, it was found that ischemic conditioning (IC) upon reperfusion of the heart (ischemic postconditioning, IPostC) or applied to a remote tissue or organ (remote ischemic conditioning, RIC) had a similar protective effect on the myocardium [8][9]. Following the heart, (R)IC was also described for various other organs including the liver, brain, lungs, and kidney [10][11][12][13]. In addition, several non-ischemic stimuli (such as hyperthermia or transient pacing) and pharmacological substances (such as erythropoietin and nicorandil) were also found to confer cellular tolerance to a major ischemic period by underlying mechanisms similar to those mediating IC [14][15][16][17][18][19][20][21]. This is also the case for some of the generally used anesthetics agents, which is called anesthetic conditioning (AC). AC is particularly attributed to volatile anesthetic (VA) agents, such as sevoflurane, isoflurane and desflurane, and to a lesser extent to the intravenous anesthetic agent propofol. Next to this, VA affect various cells of the immune system, either by direct interaction of the drug with the cell or indirectly, by altering environmental conditions; hence IRI, of which many of these effects seem favorable during (kidney) transplantation. Therefore, the use of VA during the donation, transplantation, and even the preservation period, could be an attractive way to reduce injury and improve graft function.
Anesthetic agents have pleiotropic effects, meaning that there are effects other than inducing anesthesia and analgesia, which affect many cells and systems in our body. This was already reported in the beginning of the 20th century. In 1911, Gaylord and Simpson showed that breast cancer cells grew more rapidly in mice when exposed to ether or chloroform [22]. A few years later, in 1916, Graham reported that leucocytes exposed to ether showed impaired phagocytosis of streptococci [23]. After these early findings, it was quiet in this area of research for a long period of time, but in the past few decades there has been a growing interest in the effects of our generally used anesthetic and analgesic agents on various organs, the immune system, and phenomena such as IRI. Experiments on pulmonary epithelial and endothelial cells suggest that the trifluoronated carbon groups, which are part of all modern VA, are responsible for the anti-inflammatory and immunomodulatory effects [24].
The first paper describing the advantageous effects of VA on IRI dates back to 1976, in which Bland and Lowenstein reported the protective properties of halothane on the myocardium in an ischemia and reperfusion (I/R) model of a canine heart [25]. In 1988, Warltier et al. showed that the recovery of cardiac function after exposing the myocardium to a brief period of ischemia was enhanced by isoflurane. Dogs anesthetized with isoflurane regained full contractile performance within two hours after reperfusion compared to control animals, which after five hours regained only 50% of their baseline contractile performance [26]. Since then, numerous animal studies and clinical trials have been published reporting the cardioprotective properties of VA against IRI and elucidating mechanistic pathways. Several meta-analyses have been performed comparing the use of VA versus propofol on cardiac and patient outcome in cardiac surgery, with results ranging from no or minor difference (e.g., lower cardiac-troponin-1,cTn1) to a reduction in cardiac complications, inotropic requirements, duration of mechanical ventilation, and mortality in the advantages of VA [27][28][29]. Modes of application of the VA in these studies ranged from specific pre- or postconditioning protocols to administration of the VA during the entire surgical procedure. The most recent and largest meta-analysis, performed by Bonnani et al. including 42 trials and 8197 patients, however, showed that VA are superior to propofol on long term mortality and postoperative morbidity [30]. In their analysis, the use of VA in patients undergoing cardiac surgery with the use of cardiac pulmonary bypass was associated with a lower one-year mortality, myocardial infarction, lower cTnT release, less need for inotropic support, shorter extubation time, and higher cardiac index/cardiac output compared to the use of propofol, indicating a myocardial protective effect of VA in this setting [30].
In addition to the heart, protective effects of VA are also described for other organs such as the lungs, liver, brain, and kidney, both in vitro and in vivo in different animal species [31][32][33][34][35][36][37][38][39][40][41]. Regarding the kidney, evidence of protection is either indirect or restricted to animal work. In rats, Lee et al. showed that clinically relevant concentrations (1 minimal alveolar concentration, MAC) of VA (sevoflurane and isoflurane) administered both during and after renal ischemia conferred profound protection against renal IRI, resulting in dramatically lower plasma creatinin levels and reduced renal necrosis 24–72 h after injury, compared with rats that received the intravenous agents pentobarbital or ketamine [42]. In mice subjected to renal I/R, anesthesia with isoflurane led to reduced infiltration of neutrophils, macrophages and lymphocytes post-reperfusion compared to mice anesthetized with pentobarbital [43]. Ko et al. studied the impact of choice of anesthetic agent on a variety of renal and hepatic function markers in patients undergoing hemi-hepatectomy [44]. Typically, during this procedure, very conservative fluid administration regimes are used. While this may achieve the aim of limiting intraoperative blood loss, it also jeopardizes renal function if significant renal hypoperfusion and ischemia occurs. In this study, patients who received desflurane had significantly better creatinine and glomerular filtration rate (GFR) values on the first day after surgery than patients anesthetized with propofol.
Mechanistically, protection against IRI with the use of VA most likely is not conferred by the activation of one single pathway. VA have been shown to interact with many of pathophysiological processes involved in IRI. These points of engagement are outlined below and summarized in Figure 1.
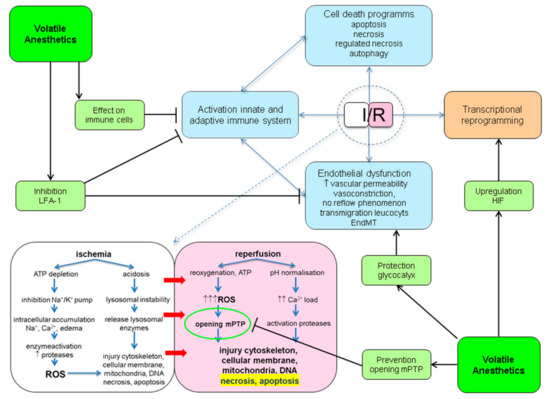
Figure 1. Potential protective points of engagement of volatile anesthetics (VA) in ischemia reperfusion injury (IRI). ATP: adenosine triphosphate; EndMT: endothelial to mesenchymal transition; HIF: hypoxic inducible factor; I/R: ischemia/reperfusion; LFA-1: lymphocyte function antigen-1; mPTP: mitochondrial permeability transition pore; ROS: reactive oxygen species.
Only a few clinical trials have looked into the effect of anesthetics used in organ procurement procedures and kidney transplantation on graft outcomes. In a retrospective cohort analysis, Perez-Protto et al. studied the impact of VA during DBD donor organ procurement on graft survival in recipients (preconditioning) and compared VA-exposed (n = 138) to non-VA-exposed (n = 75, no anesthetic, etc.) donors [45]. They found no significant differences between the groups in 30-day and five-year graft survival of heart, liver, lung, and kidney transplants. In this analysis, however, sample size was relatively small, and the rates of graft failures in both groups were low (25 of the 446 transplanted organs, 5.6%); consequently, there was a lack of power to confirm conclusions. In addition, the dosage and duration of exposure of the VA were unclear. In prospective cohort analysis, Lee et al. compared kidney function in recipients of living donor kidneys according to the type of anesthetic used in their matching donor (preconditioning) and found no differences between desflurane (n = 50) and propofol (n = 49) [46]. Changes in serum creatinine post-transplantation and estimated glomerular filtration rate (eGFR) at day of discharge were comparable between groups. In a pilot proof of concept study of the Volatile Anesthetic Protection of Renal transplants (VAPOR) project of our group, we compared a sevoflurane-based anesthesia with a propofol-based anesthesia in living donor kidney transplantation (LDKT) (VAPOR-1 study; pre- and post-conditioning) [47]. Although this specific setting of kidney transplantation is associated with significantly less IRI, low incidence of DGF (<5%) and better graft outcome compared to deceased donor kidney transplantation, it provides an elegant research model due to a homogeneous model of IRI and reproducible ischemia times. In addition, this model provides the possibility to treat the donor. The primary outcome measure was a set of urinary biomarkers consisting of kidney injury molecule-1 (KIM-1), N-acetyl-D-glucosaminidase (NAG), and heart-type fatty acid binding protein (H-FABP) reflecting kidney injury with the hypothesis of higher levels in propofol-treated patients. Whereas H-FABP was highest in the first urine sample upon reperfusion and levels were comparable between treatment groups, levels of KIM-1 and NAG increased again the first day after transplantation after an initial decrease. To our surprise, sevoflurane-treated patients showed higher levels of KIM-1 (second day) and NAG (first day, second day) after transplantation compared to propofol-treated patients. This, however, was not associated with inferior graft outcome [48]. In contrast, higher KIM-1 levels the first day after transplantation were significantly associated with better eGFR one month post-transplantation (R = 0.412, p = 0.002), which was almost the case for KIM-1 levels measured the second day after transplantation (p = 0.074). This could be due to the opposed dual role of KIM-1, with evidence that in acute renal injury, KIM-1 plays a role in the regeneration and repair process. AKI surviving PTC expressing KIM-1 are able to phagocytize luminal cellular debris consisting of apoptotic and necrotic cells, enabling the PTC to down-regulate the innate immune response upon AKI, which could be beneficial in kidney transplantation [49][50]. Higher NAG levels (first and second in this case) might be a reflection of regenerated tubular cells showing baseline lysosomal activity rather than a reflection of injury. An intriguing outcome of our study was the difference in incidence of T cell mediated rejection during a two-year follow-up in favor of sevoflurane-treated patients. This, however, was not our primary outcome measure, and the number of events and patients (n = 9, n = 57, respectively) did not allow us to perform a multivariate analysis. Well-designed randomized controlled trials (RCTs) need to be performed to see whether the effects of VA are clinically relevant and are attributable to improved outcome after kidney transplantation. One can hypothesize that the potential beneficial effects are more pronounced in the setting of deceased donor kidney transplantation, because the extent of IRI in this setting exceeds the amount encountered in LDKT. Currently, VAPOR-2, an international multicenter RCT (ClinicalTrials.gov: NCT02727296), in which 488 patients receiving a DBD or DCD kidney will be randomized to a sevoflurane–remifentanil-based anesthesia or a propofol–remifentanil-based anesthesia (post-conditioning), is running. The primary outcome in this study is DGF, an adequate reflection of IRI. After completion of the VAPOR project, we hope to be able to answer the question as to whether the choice of anesthetics contributes to improved graft and patient outcome after kidney transplantation.
Which type of VA confers the most protective effect is disputed. In the animal experiments of Lee et al., isoflurane and sevoflurane showed comparable effects but were significantly more profound than desflurane [42]. This could be due to the fact that desflurane has a lower lipid solubility. Interaction of the VA with the bi-lipid membranes of cells and organelles has been proposed as a potential point of engagement of the protective effects of these agents. Park et al. retrospectively compared the effect of desflurane (n = 71) and sevoflurane (n = 73) in living kidney donors and their matching recipients (pre- and post-conditioning). They found no differences between both anesthetics in terms of eGFR, creatinine and blood urea nitrogen (BUN) one year post-transplantation, as was the case for DGF, AR and graft failure [51]. Similar findings were found by Savran Karadeniz et al., in a prospective cohort analysis comparing desflurane (n = 30) and sevoflurane (n = 35) [52]. Although sevoflurane anesthesia was associated with higher IL-8 levels one day and one week after transplantation, both agents had similar effects on graft function [52].